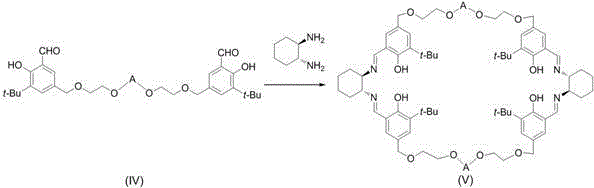
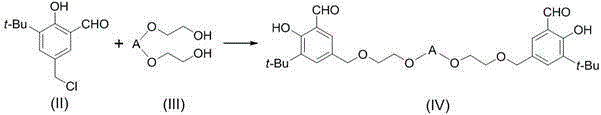
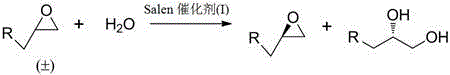Trivalent cobalt Salen catalyst, synthesis method as well as application of trivalent cobalt Salen catalyst to resolution of terminal epoxides
A synthesis method and epoxide technology, which are used in the preparation of cobalt organic compounds, organic compounds, chemical instruments and methods, etc., can solve problems such as unfavorable large-scale industrial production, poor catalyst stability, and complex catalyst synthesis, and achieve excellent disassembly. The effect of splitting effect, good stability and high splitting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Trivalent cobalt Salen catalyst (I)-a (-O-A-O- is pyrocatechol ether, X is the synthetic steps of acetate):
[0045] (1) Preparation of compound (III)-a
[0046]
[0047]Under nitrogen protection, compound (III)-a (3.96g, 20mmol), sodium hydride (3.52g, 88mmol) and anhydrous tetrahydrofuran (20ml) were added to a 150ml dry three-necked flask, and stirred at 25°C for 1 hour. Compound (II) (9.07 g, 40 mmol) was dissolved in anhydrous tetrahydrofuran (50 ml), slowly dropped into the reaction flask, and stirring was continued for 20 hours after the drop was completed. After the reaction was stopped, most of the solvent was distilled off under reduced pressure, ethyl acetate (40ml) and 5% dilute hydrochloric acid (40ml) were added, the organic phase was separated, the aqueous phase was extracted three times with ethyl acetate, and the organic phase was combined with anhydrous sulfuric acid Dry over sodium, filter and concentrate, and the residue is separated by silica ge...
Embodiment 2
[0055] The synthetic steps of trivalent cobalt Salen catalyst (I)-b (-O-A-O- is 5-chloro-resorcinol ether, X is acetate):
[0056] (1) Preparation of compound (IV)-b
[0057]
[0058] Under nitrogen protection, compound (III)-b (3.96g, 20mmol), potassium tert-butoxide (11.20g, 100mmol) and anhydrous acetonitrile (20ml) were added to a 150ml dry three-necked flask, and stirred at 20°C for 2 hours . Compound (II) (9.07 g, 40 mmol) was dissolved in anhydrous acetonitrile (50 ml), slowly dropped into the reaction flask, and stirring was continued for 22 hours after the drop was completed. After the reaction was stopped, most of the solvent was distilled off under reduced pressure, ethyl acetate (40ml) and 5% dilute hydrochloric acid (40ml) were added, the organic phase was separated, the aqueous phase was extracted three times with ethyl acetate, and the organic phase was combined with anhydrous sulfuric acid Dry over sodium, filter and concentrate, and the residue is separat...
Embodiment 3
[0066] The synthetic steps of trivalent cobalt Salen catalyst (I)-c (-O-A-O- is hydroquinone ether, X is a trifluoromethanesulfonate radical):
[0067] (1) Preparation of compound (IV)-c
[0068]
[0069] Under nitrogen protection, compound (III)-c (3.96g, 20mmol), anhydrous potassium carbonate (12.42g, 90mmol) and anhydrous ether (20ml) were added to a 150ml dry three-necked flask, and stirred at 30°C for 2 hours . Compound (II) (9.07 g, 40 mmol) was dissolved in anhydrous diethyl ether (50 ml), slowly dropped into the reaction flask, and stirring was continued for 20 hours after the drop was completed. After the reaction was stopped, most of the solvent was distilled off under reduced pressure, ethyl acetate (40ml) and 5% dilute hydrochloric acid (40ml) were added, the organic phase was separated, the aqueous phase was extracted three times with ethyl acetate, and the organic phase was combined with anhydrous sulfuric acid It was dried over sodium, filtered and concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com