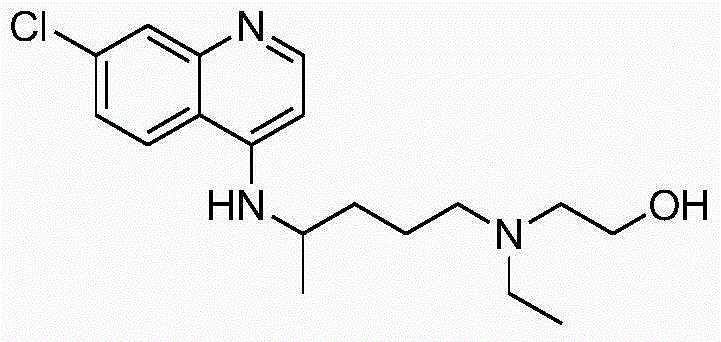
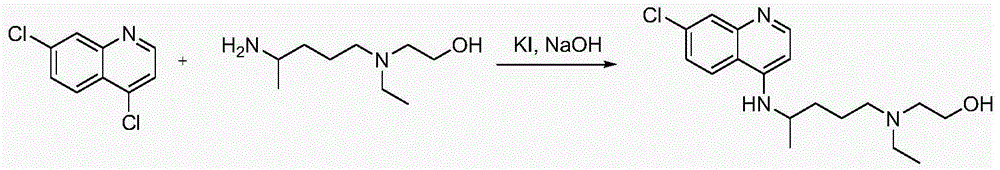
Synthetic method of 5-(N-ethyl-N-2-ethylol amine)-2-amylamine
A technology of hydroxyethylamine and a synthesis method, which is applied in the synthesis field of 5--2-pentylamine, can solve the problems of low purity of target product, low total yield and the like, and achieves easy industrial production, high conversion rate of raw materials, Avoid the effect of separation operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A kind of synthetic method of 5-(N-ethyl-N-2-hydroxyethylamine)-2-pentylamine, its step is as follows:
[0027] (1) Preparation of catalyst: Disperse and dissolve 0.5g or 20 or 40 or 60 or 80 or 100g of cobalt nitrate into 100ml or 1 or 2L of ethanol, then add 5g or 50 or 200 or 500 or 1000 or 1500 or 2000g of oxidized Magnesium nanopowder, stirred and ultrasonicated at 40 or 45 or 50 or 59 Hz for 10 or 30 or 60 minutes, evaporated the ethanol solvent to dryness, and vacuum dried at 80 or 90 or 100 or 110 or 120°C for 1 or 3 or 5 Or 10 hours, and finally grind into a fine powder with a particle size of 0.5-2 microns to obtain 5-chloro-2-pentanone and N-ethylethanolamine to directly react to prepare 5-(N-ethyl-N-2-hydroxyethyl Catalyst for the reaction of amine)-2-pentanone.
[0028] (2) Add 1.0 or 1.3 or 1.5L of xylene, 320g or 325 or 330g of 5-chloro-2-pentanone and 470 or 480 or 490g of N-ethylethanolamine in turn in the reaction flask, start stirring, add 1.5 Or 3 ...
Embodiment 2
[0031] A kind of synthetic method of 5-(N-ethyl-N-2-hydroxyethylamine)-2-pentylamine, its step is as follows:
[0032] (1) Preparation of catalyst: Disperse and dissolve 0.5g or 25 or 45 or 65 or 85 or 100g of nickel nitrate into 100ml or 1 or 2L of ethanol, then add 5g or 60 or 220 or 600 or 1100 or 1600 or 2000g of oxidized Magnesium nanopowder, stirred and ultrasonicated at 40 or 44 or 51 or 59 Hz for 10 or 25 or 35 or 60 minutes, evaporated the ethanol solvent, and vacuum dried at 80 or 93 or 101 or 110 or 120°C for 1 or 3 Or 5 or 10 hours, and finally grind into a fine powder with a particle size of 0.5-2 microns to obtain 5-chloro-2-pentanone and N-ethylethanolamine to directly react to prepare 5-(N-ethyl-N-2- Catalyst for the reaction of hydroxyethylamine)-2-pentanone.
[0033] (2), sequentially add 1.0 or 1.2 or 1.5L xylene, 320 or 326 or 330g 5-chloro-2-pentanone and 470 or 482 or 490g N-ethylethanolamine in the reaction flask, start stirring, add 1.5 Or 4 or 6 or 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com