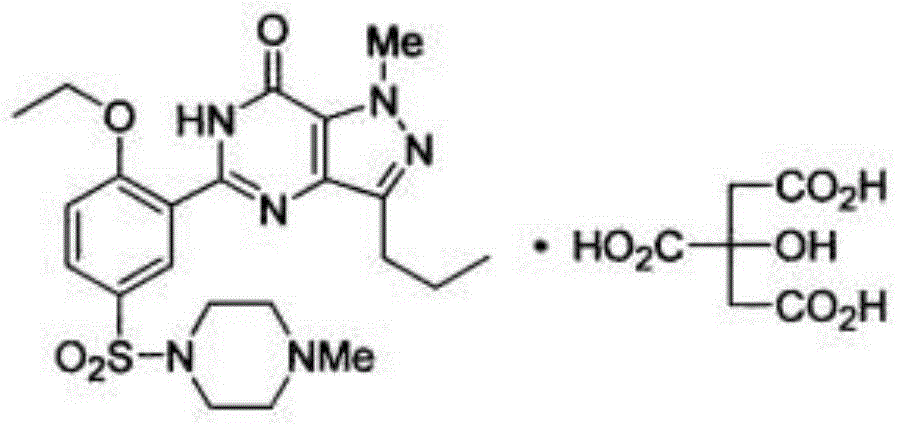
Sildenafil citrate compound and pharmaceutical composition thereof
A technology of sildenafil citrate and compound, applied in the field of sildenafil citrate compound and pharmaceutical composition thereof, can solve problems such as slow drug dissolution rate, problems in drug absorption rate or degree, poor water solubility and the like , to achieve the effect of improving solubility and hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
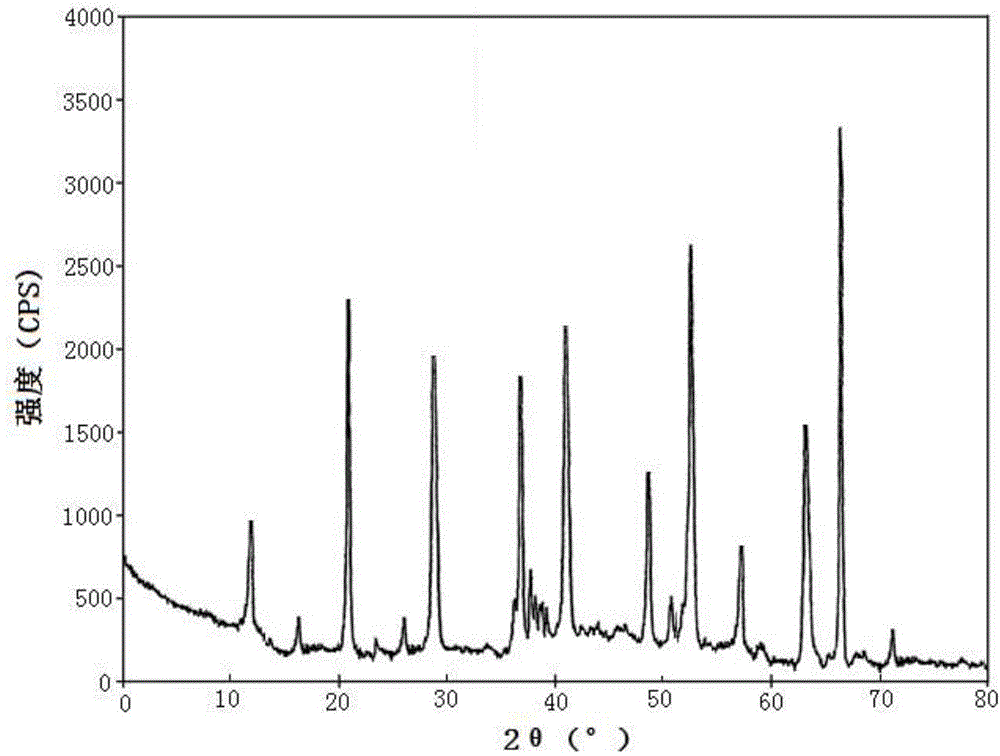
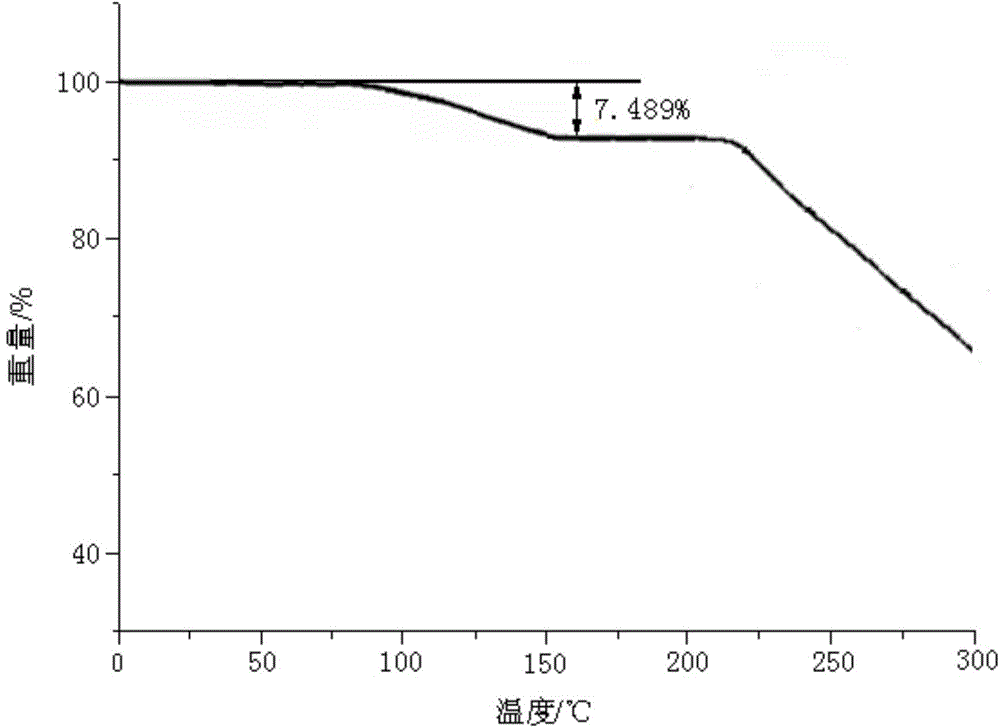
[0056] [Example 1] Preparation of Sildenafil Citrate Compound
[0057] 1) Sample dissolution: Dissolve 1 kg of sildenafil citrate in a mixed solution of 5L dimethylformamide and acetone at 45°C (the volume ratio of dimethylformamide and acetone in the mixed solution is 3: In 1), solution I was obtained, and the temperature of solution I was kept at 38° C.;
[0058]2) Crystallization: take solution I and press filter to remove insoluble matter to obtain solution II, keep the temperature of solution II at 38°C, under stirring, add 1 / 4 of the volume of solution II pure water dropwise continuously, and control the addition within 1.5 hours; After 1.5 hours of crystallization, continue to add 3 / 4 of the volume of solution II pure water dropwise under stirring, control the addition within 1 hour, and stir until crystallization; after 1 hour of crystal growth, cool down to 15°C and keep it warm for 3 hours. Centrifuge to collect the sildenafil citrate wet product;
[0059] 3) Water...
preparation Embodiment 1
[0069] [Preparation Example 1] Sildenafil Citrate Tablets
[0070] Prescription: 25mg
[0071]
[0072] Preparation:
[0073] 1. Screening: the raw and auxiliary materials are all passed through a 100-mesh sieve;
[0074] 2. Weigh sildenafil citrate, microcrystalline cellulose, lactose, calcium hydrogen phosphate, and croscarmellose sodium according to the prescription, and mix well;
[0075] 3. Granulation: make soft material with 2.5% hypromellose aqueous solution, granulate with 18 mesh sieve, dry at 55℃~65℃ for 3.0-3.5h, granulate with 18 mesh sieve;
[0076] 4. Total blending: add croscarmellose sodium and magnesium stearate according to the prescription amount;
[0077] 5. Intermediate product detection: determine the content of sildenafil, and calculate the weight of the tablet to be compressed;
[0078] 6. Tablet pressing: use Φ6.5mm / Φ8.5mm / Φ11mm shallow concave punching die to press the tablet respectively;
[0079] 7. Film coating, coating weight gain 2-4%; ...
preparation Embodiment 2
[0081] [Preparation Example 2] Sildenafil Citrate Tablets
[0082] Prescription: 50mg
[0083]
[0084]
[0085] Preparation method: same as preparation example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com