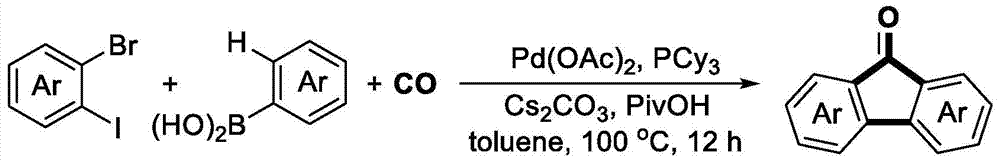
Preparation method for 9-fluorenone and T type oligomeric phenylene skeleton compounds
A compound, polyphenylene technology, applied in the field of preparation of 9-fluorenones and their T-type oligophenylene skeleton compounds, can solve problems such as harsh reaction conditions, and achieve the effect of mild conditions and high-efficiency preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
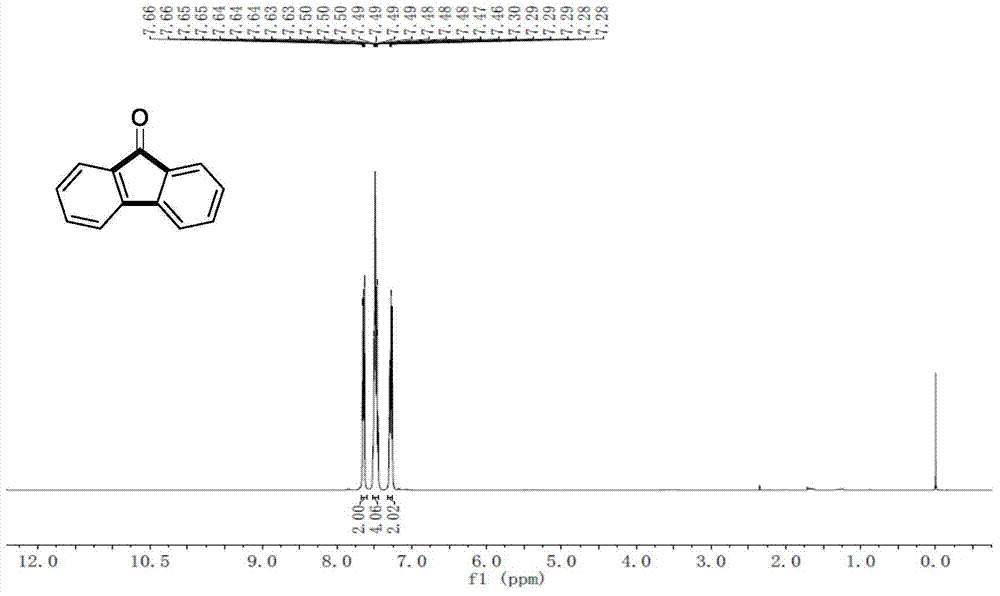
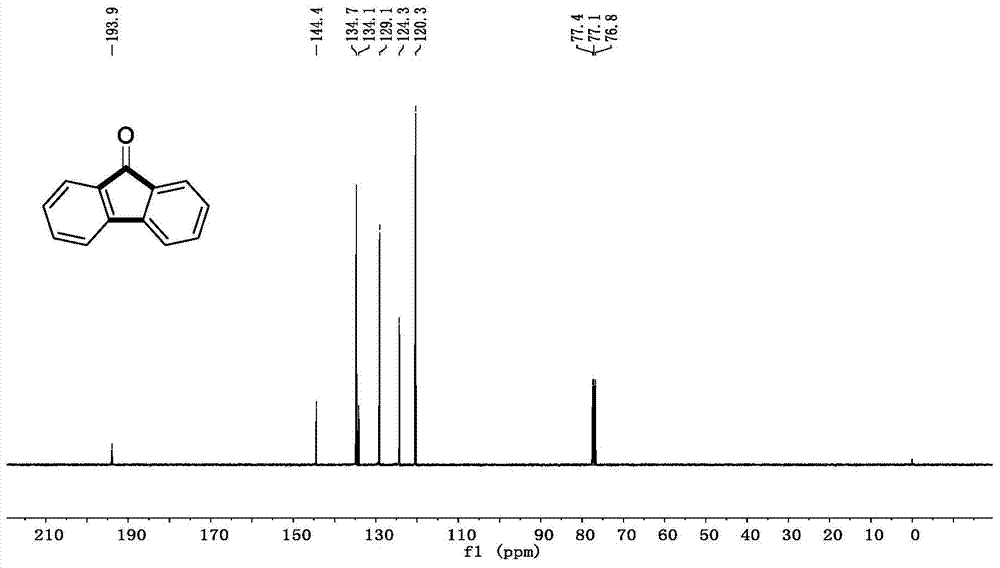
[0067] Example 1: Under nitrogen protection, add phenylboronic acid (0.6mmol, 73.2mg), cesium carbonate (1.5mmol, 489mg), palladium acetate (0.025mmol, 5.6mg) in a Schlenk reaction tube, and place in glove box Add tricyclohexylphosphine (0.05mmol, 14mg), take it out and replace it with CO gas atmosphere, add o-bromoiodobenzene (0.5mmol, 64.2uL) and pivalic acid (0.5mmol, 51mg) to the system successively, and then After adding 3 ml of toluene and stirring for 5 minutes, the reaction was placed in an oil bath at 100° C. for 12 hours. After the reaction was over, 3 milliliters of saturated ammonium chloride solution was added to the system to quench the reaction, and 15 milliliters of ethyl acetate was added for extraction three times. The organic phases were combined, dried over anhydrous sodium sulfate, and separated by column chromatography to obtain the product. The yield was 90% (see Table 1 for details).
Embodiment 2-13
[0068] Embodiment 2-13: Except for using different aryl boronic acids, other reaction conditions are the same, specifically as follows:
[0069] Under nitrogen protection, add arylboronic acid (0.6mmol), cesium carbonate (1.5mmol, 489mg), palladium acetate (0.025mmol, 5.6mg) in a Schlenk reaction tube, and place in a glove box, add tricyclohexylphosphine (0.05mmol, 14mg), after taking out, replace it with CO gas atmosphere, add o-bromoiodobenzene (0.5mmol, 64.2uL) successively in the system, pivalic acid (0.5mmol, 51mg), then add 3 milliliters of toluene, stir 5 Minutes later, the reaction was placed in an oil bath at 100°C for 12 hours. After the reaction was over, add 3 milliliters of saturated ammonium chloride solution to the system to quench the reaction, add 15 milliliters of ethyl acetate for extraction three times, combine the organic phases, dry with anhydrous sodium sulfate, and separate the product by column chromatography (see Table 1).
Embodiment 14
[0070] Example 14: Under nitrogen protection, 9,9 dioctylfluorene-1,3,2-propanol borate (0.6mmol, 262mg), cesium carbonate (1.5mmol, 489mg) were added to a Schlenk reaction tube, Palladium acetate (0.025mmol, 5.6mg), and placed in the glove box, added tricyclohexylphosphine (0.05mmol, 14mg), replaced with CO gas atmosphere after taking it out, and added o-bromoiodobenzene (0.5mmol, 64.2uL), pivalic acid (0.5mmol, 51mg), then 3ml of toluene was added, and after stirring for 5 minutes, the reaction was placed in an oil bath at 100°C for 12 hours. After the reaction was over, 3 milliliters of saturated ammonium chloride solution was added to the system to quench the reaction, and 15 milliliters of ethyl acetate was added for extraction three times. The organic phases were combined, dried over anhydrous sodium sulfate, and separated by column chromatography to obtain the product. The rate was 45% (see Table 1 for details).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com