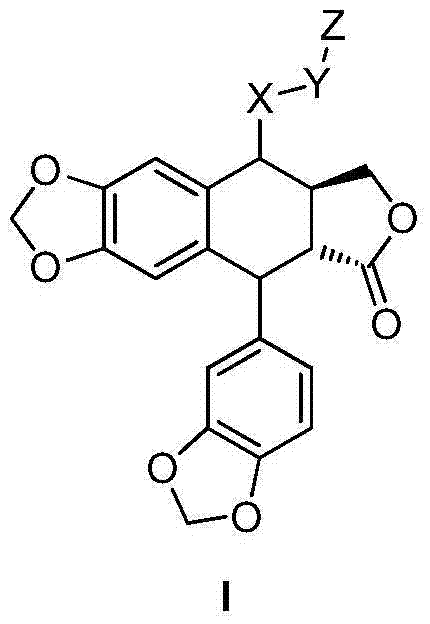
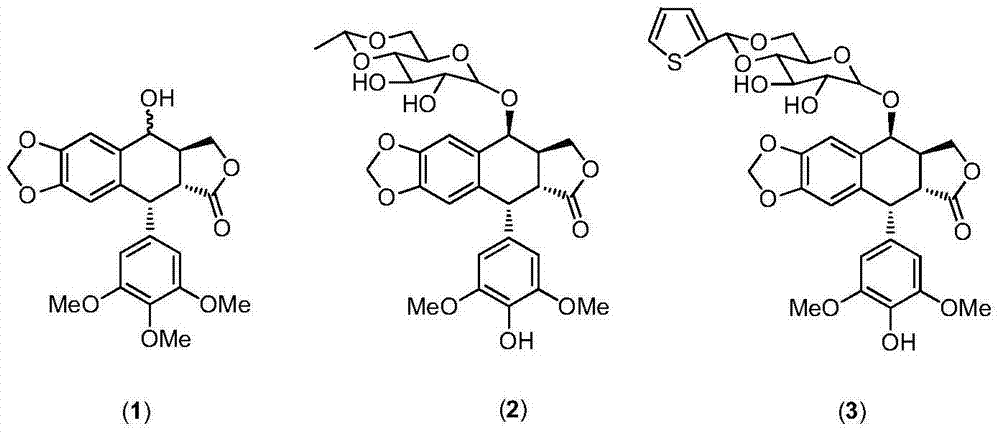
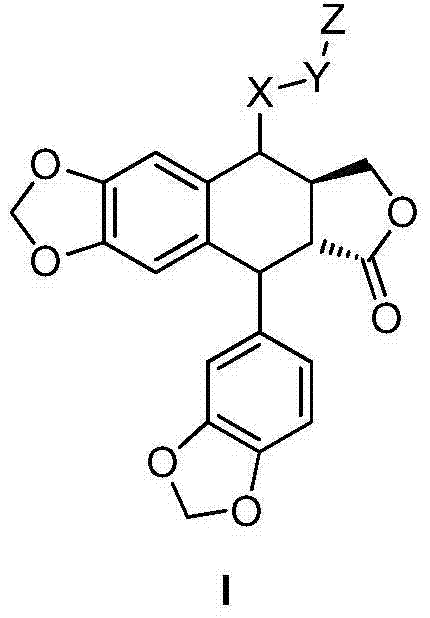
Otobain compound, preparation method therefor and application thereof
A technology of myristin and ottoman, applied in drug combination, organic chemistry, pharmaceutical formula, etc., can solve the problems of high cost, difficulty in the total synthesis of podophyllotoxin, and many steps, and achieve low toxicity and good anti-tumor Active, less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Compound 2
[0036] Add 15.00g of piperonal into 150mL of dichloromethane, stir until dissolved, then add 1.90g of p-toluenesulfonic acid and 11.02mL of 1,3-propanedithiol, under nitrogen protection, and stir at room temperature for 24 hours. Add saturated sodium carbonate and stir for 2 hours, separate the organic layer, wash with saturated brine, dry with anhydrous sodium sulfate, filter with suction, spin off the solvent to obtain a yellow solid, recrystallize from absolute ethanol to obtain a white needle-like solid, the yield is 90% .
Embodiment 2
[0038] Preparation of compound 3
[0039] 4.00 g of compound 2 was added to 30 mL of anhydrous tetrahydrofuran and stirred until dissolved, then the system was lowered to -78°C, and 6.96 mL of butyllithium was added dropwise, and the reaction was continued for 1 hour. A solution of 1.44 g of 2(5H)-furanone and 12 mL of tetrahydrofuran was added dropwise, and the reaction was continued for 2 hours. After adding 12 mL of acetic acid dropwise, it was slowly raised to room temperature and reacted for another 3 hours. The tetrahydrofuran was removed by rotary evaporation, extracted with dichloromethane, washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was spun off to obtain a brown-yellow oil, which was recrystallized from ethyl acetate to obtain a white solid with a yield of 80%. Melting point: 162-163°C; 1 H NMR (500MHz, CDCl 3 ):δ1.85-1.99(m,2H),2.40-2.46(m,1H),2.66-2.76(m,4H),2.82-2.87(m,1H),2.98-3.04(m,1H),4.18 -4.22 (m, 1H), 4.39-4.43 (m, ...
Embodiment 3
[0041] Preparation of Compound 4
[0042] Add 1.20mL of diisopropylamine to 5.00mL of anhydrous tetrahydrofuran and stir well, lower the system to -30°C, add 3.20mL of butyllithium dropwise, continue the reaction for 30 minutes, then raise to room temperature for 60 minutes. The system was lowered to -78°C again, and a solution of 2.00 g of compound 3 and 15 mL of tetrahydrofuran was added dropwise, and the reaction was continued for 2 hours. A solution of 1.11 g of piperonal and 5 mL of anhydrous tetrahydrofuran was added dropwise, reacted for 1 hour, and slowly rose to room temperature for reaction for another 3 hours. After adding dropwise 1 mL of acetic acid, tetrahydrofuran was removed by rotary evaporation, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, and spinned to remove the solvent, followed by column chromatography (ethyl acetate / petroleum ether, 3:7) to obtain compound 4(42 %), white solid, melting point: 181-182°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com