Method for preparing six fluorine phosphonic acid ester base fe organism derivatives
A technology of diethyl difluoromethylphosphonate and diethyl bromodifluoromethylphosphonate is applied in the fields of organic chemical industry and fine chemical industry, and can solve the problems of toxic and unstable free radical initiators, and achieve high application Value, convenient separation and purification, and the effect of less waste discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
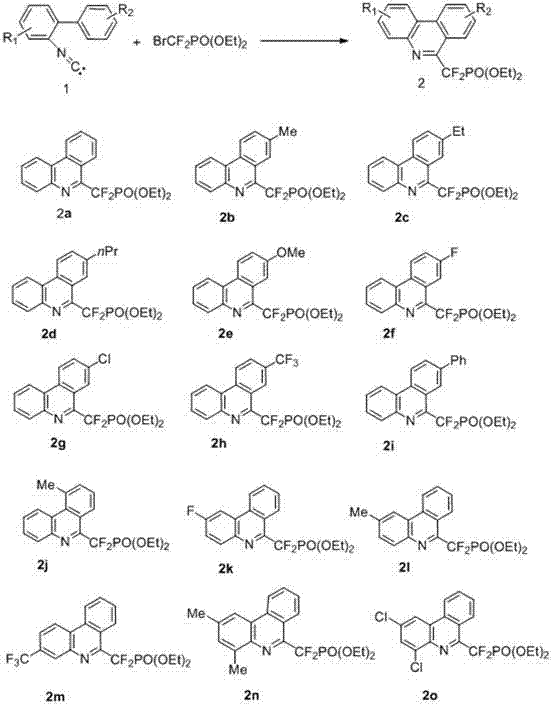
[0026] Synthesis of diethyl difluoro(phenanthridin-6-yl)methylphosphonate (2a)
[0027] Weigh 2-phenylbenzene isocyanide 1a (1mmol), ethyl bromodifluorophosphonate (4.0mmol), three (2-phenylpyridine) iridium (0.05mmol) and sodium bicarbonate (2.0mmol), and Add it to a 25mL Schlenk reaction flask in turn, then add acetonitrile (10.0mL) and place it under a 20W LED lamp to react for 40h. After the reaction is over, remove the solvent under reduced pressure and use petroleum ether / ethyl acetate as the eluent , separated on a silica gel column, the yield of the product diethyl difluoro(phenanthridin-6-yl)methylphosphonate was 78%.
[0028] 1 H NMR (400 MHz, CDCl 3 ): δ = 8.66 (d, J = 7.6 Hz, 1H), 8.59 (d, J = 7.6 Hz, 2H), 8.23 (d, J = 7.2 Hz, 1H), 7.87 (t, J = 7.2 Hz, 1H), 7.69-7.79 (m, 3H), 4.45-4.62 (m, 4H),1.42 (t, J = 7.2 Hz, 6H); 13 C NMR (125 MHz, CDCl 3 ): δ = 150.5 (td, J 1 = 25.4, J 2 = 13.3 Hz), 141.7, 133.9, 131.1, 130.5, 129.0, 128.9, 127.7, 126.8,...
Embodiment 2
[0030] Synthesis of diethyl difluoro(8-methylphenanthridin-6-yl)methylphosphonate (2b)
[0031] Weigh 2-p-tolylbenzonitrile 1b (0.5mmol), ethyl bromodifluorophosphonate (1.0mmol), tris(2-phenylpyridine) iridium (0.02mmol) and sodium carbonate (1.0mmol), And sequentially added in the 25mL Schlenk reaction bottle, then, add dichloromethane (5.0mL), and place to react 48h under the blue LED lamp of 5 watts, after the reaction finishes, remove solvent under reduced pressure, use sherwood oil / ethyl acetate as The eluent was separated on a silica gel column, and the yield of the product diethyl difluoro(8-methylphenanthridin-6-yl)methylphosphonate was 76%.
[0032] 1H NMR (400 MHz, CDCl 3 ): δ = 8.54 (d, J = 8.0 Hz, 2H), 8.20 (d, J = 8.0 Hz, 1H), 8.36(s, 1H), 7.68-7.76 (m, 3H), 4.51-4.59 (m, 4H),2.61 (s, 3H), 1.42 (t, J = 7.2 Hz, 6H); 13 C NMR (125MHz, CDCl 3 ): δ = 150.2 (td, J 1 = 26.0, J 2 = 14.2 Hz), 141.4, 137.8, 132.9, 131.8, 130.4, 128.8, 128.5, 127.4, 126.0,...
Embodiment 3
[0034] Synthesis of diethyl (8-ethylphenanthridin-6-yl)difluoromethylphosphonate (2c)
[0035] Weigh 2-p-ethylphenylisocyanate 1c (0.5mmol), ethyl bromodifluorophosphonate (2.0mmol), [4,4'-di-tert-butyl-2,2'-bipyridine] di [2-2-Phenylpyridine] Iridium hexafluorophosphate (0.01mmol) and triethylamine (2.0mmol) were added successively to a 25mL Schlenk reaction flask, then tetrahydrofuran (5.0mL) was added, and placed React under a 40-watt LED lamp for 36 hours. After the reaction, remove the solvent under reduced pressure, use petroleum ether / ethyl acetate as the eluent, and separate on a silica gel column to obtain the yield of the product diethyl (8-ethylphenanthridin-6-yl) difluoromethylphosphonate was 72%.
[0036] 1 H NMR (400 MHz, CDCl 3 ): δ = 8.51-8.59 (m, 2H), 8.39 (s, 1H), 8.21(d, J = 8.0 Hz, 1H), 7.67-7.78 (m, 3H), 4.50-4.59 (m, 4H),2.91 (q, J = 7.6 Hz, 2H), 1.42 (t, J = 7.2 Hz, 6H), 1.37 (t, J = 7.6 Hz, 3H); 13 C NMR (125MHz, CDCl 3 ): δ = 150.3 (td, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com