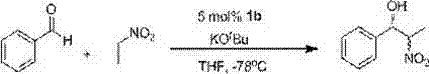
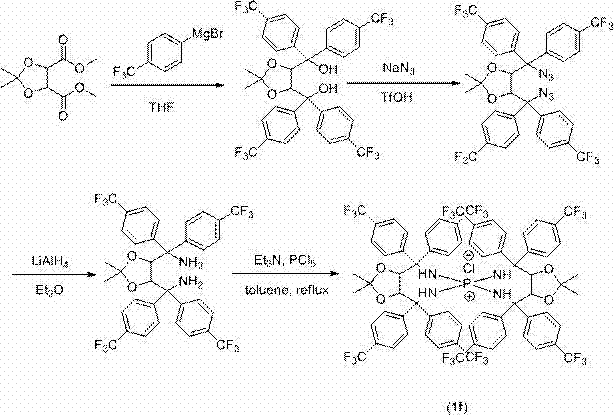
Quaternary phosphonium salt compound and preparation method thereof
A technology for quaternary phosphonium salts and compounds, which is applied in the field of quaternary phosphonium salt compounds and their preparation, can solve the problems of unreported, large amount of catalyst, slow reaction speed, etc., to improve catalytic activity and reaction stereoselectivity, and improve reaction active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: structural formula is the quaternary phosphonium salt compound shown in formula I:
[0035] where: R 1 is methyl;
[0036] The synthetic route of above-mentioned compound is as follows:
[0037]
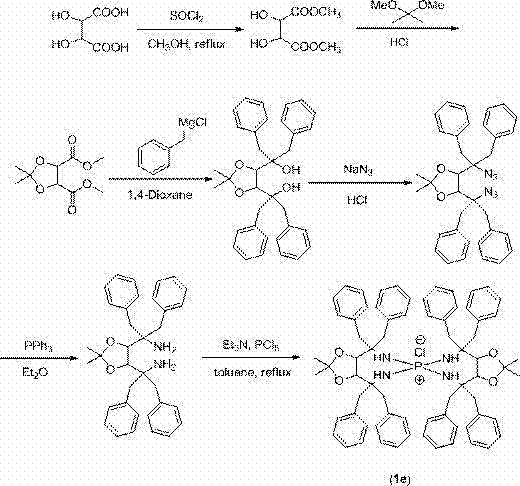
[0038](1) Add 28.5 g (190.5 mmol) L-tartaric acid into a 250 mL three-necked bottle at room temperature, then add 90 mL of methanol, stir at room temperature until the tartaric acid is completely dissolved, transfer it to an ice bath, and slowly add 55.3 mL (762 mmol) thionyl chloride, after 30 min, remove the ice bath, reflux reaction in an oil bath at 80°C for 9 h, pour the reactant into 120 mL of cold saturated sodium bicarbonate solution and vigorously stir for 30 min, separate The organic phase and the aqueous phase were extracted 3 times with ethyl acetate, the organic phases were combined, and dried over anhydrous magnesium sulfate to obtain 33.2 g of the product L-dimethyl tartrate, with a yield of 98%;
[0039] (2) Take 24 g of L-dimethyl tart...
Embodiment 2
[0048] Embodiment 2: structural formula is the quaternary phosphonium salt compound shown in formula I:
[0049] where: R 1 is phenyl;
[0050] R 1 Group is the synthetic route of the chiral quaternary phosphonium salt catalyst 1b of phenyl as follows:
[0051]
[0052] (1) At room temperature, put 28.5 g (190.0 mmol) of L-tartaric acid into a 250 mL three-neck bottle, then add 100 mL of methanol, stir at room temperature until the tartaric acid is completely dissolved, then transfer it to an ice bath, and slowly add 55.3 mL ( 762 mmol) thionyl chloride, continue to stir for 30 min, remove the ice bath, and reflux on the oil bath for 9 h; pour the reactant into 120 mL of cold sodium bicarbonate solution and stir vigorously for 30 min, then wash with acetic acid Extracted with ethyl ester and dried over anhydrous magnesium sulfate to obtain 33.14 g of the product L-dimethyl tartrate with a yield of 98%.
[0053] (2) Take 16 g (89.89 mmol) of L-dimethyl tartrate obtaine...
Embodiment 3
[0058] Embodiment 3: structural formula is the quaternary phosphonium salt compound shown in formula I:
[0059] where: R 1is n-butyl;
[0060] R 1 Group is the synthetic route of the chiral quaternary phosphonium salt catalyst 1c of n-butyl as follows:
[0061]
[0062] (1) At room temperature, put 28.5 g (190.0 mmol) of L-tartaric acid into a 250 mL three-necked bottle, then add 100 mL of methanol, stir at room temperature until the tartaric acid is completely dissolved, transfer it to an ice bath, and slowly add 68.9 mL ( 950 mmol) of thionyl chloride, continued to stir for 30 min, removed the ice bath, and refluxed on the oil bath for 9 h. The reactant was poured into 120 mL of cold sodium bicarbonate solution and vigorously stirred for 30 min, then extracted with ethyl acetate and dried over anhydrous magnesium sulfate to obtain 33.1 g of the product L-dimethyl tartrate with a yield of 98%.
[0063] (2) Take 21.72 g (122 mmol) of L-dimethyl tartrate obtained in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com