Sodium ion battery zinc sulfide based negative electrode material and preparation method thereof
A technology for sodium ion batteries and negative electrode materials, applied in battery electrodes, batteries, electrochemical generators, etc., can solve problems such as safety hazards, battery explosions, uneven deposition, etc., and achieve improved conductivity, high sodium storage capacity, and excellent The effect of the conductive network
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
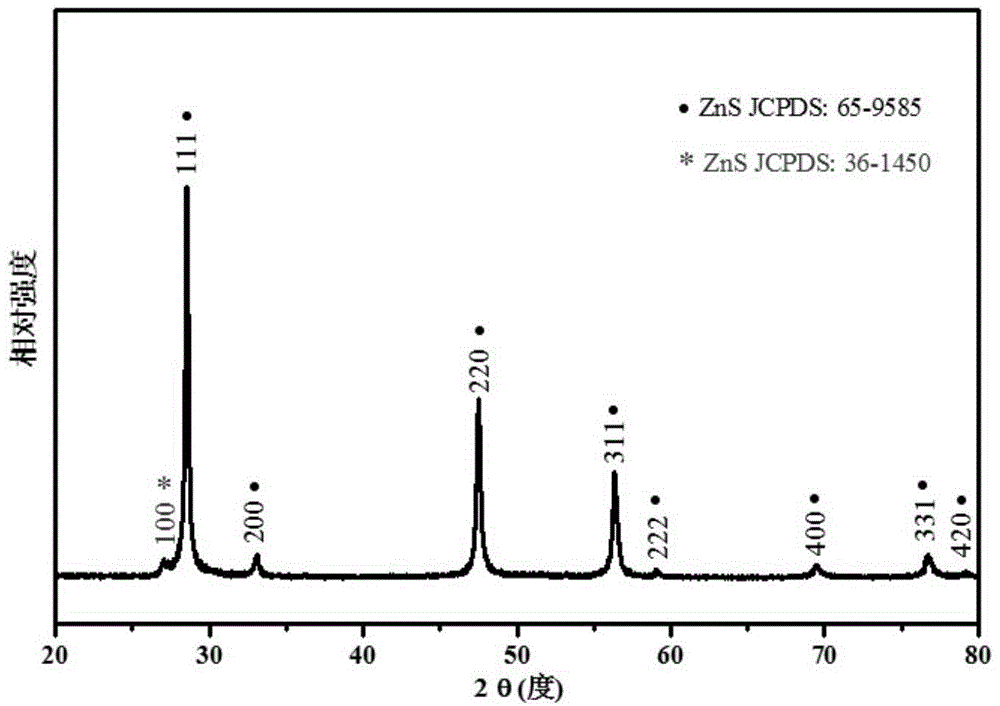
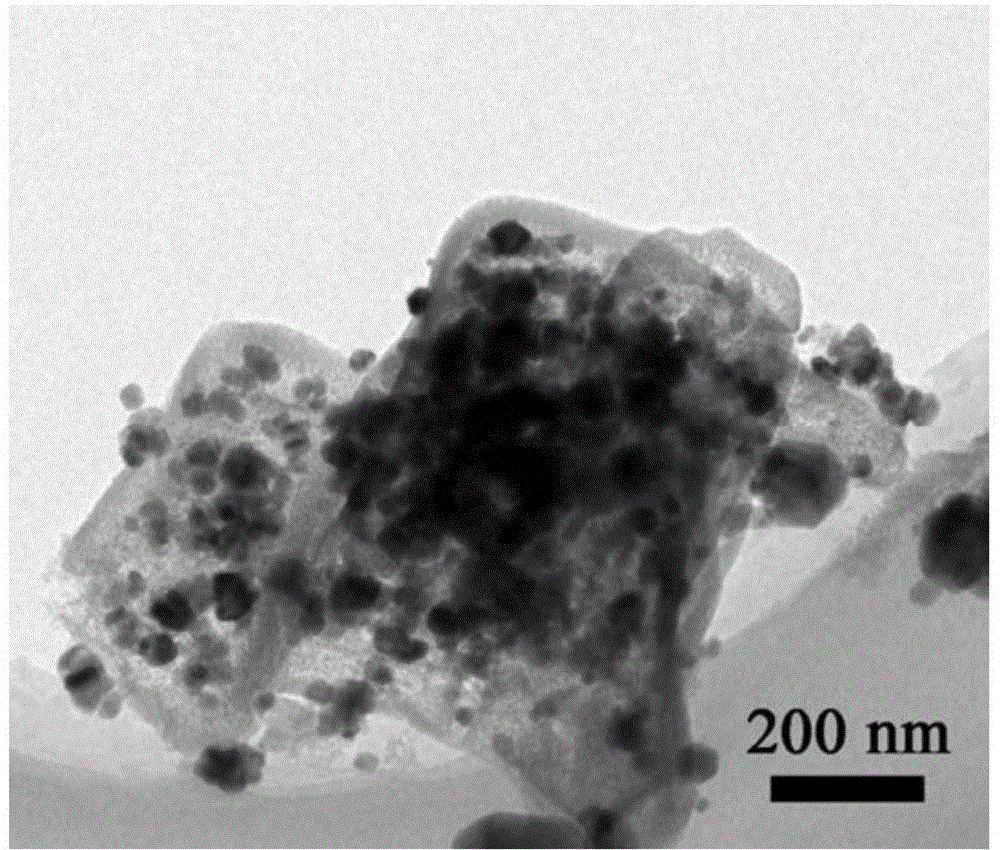
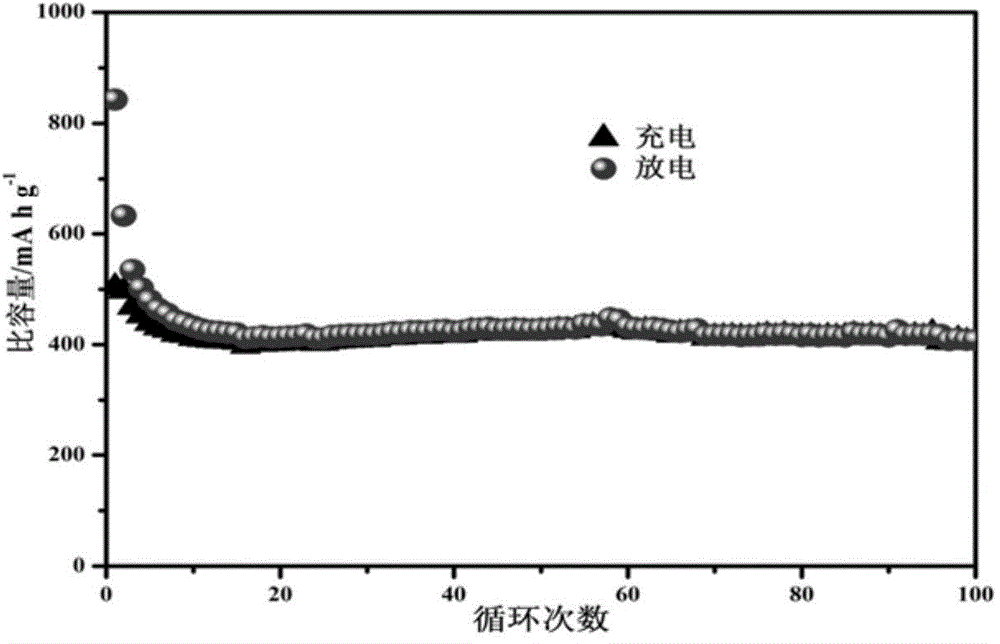
Image
Examples
Embodiment 1
[0041] Stir and dissolve 19.75g of zinc acetate dihydrate in 500ml of dimethylformamide (DMF), stir and dissolve 5.98g of terephthalic acid (BDC) in 400ml of DMF and add 8.52ml of triethylamine, mix the two solutions Stir magnetically at 20°C for 6h, obtain the precipitate by centrifugation, soak and wash with DMF, dichloromethane, and DMF in turn, and soak for 6h each time, and finally pre-dry the product at 60°C, and then vacuum at 150°C After drying for 12 hours, a zinc-based metal-organic framework was obtained. The product was calcined for 2 hours under nitrogen atmosphere, the calcining temperature was 600° C., and the heating rate was 2° C. / min.
[0042] Weigh 0.1g calcined zinc sulfide / porous carbon composite material and 0.2g thioacetamide to disperse in the mixed solution of 20ml ethylene glycol, 20ml deionized water, transfer the mixed solution to a 60ml reactor and seal it at 180 Reaction at ℃ for 24h. After the reaction, the reaction kettle was placed in the air...
Embodiment 2
[0049] Stir and dissolve 19.75g of zinc acetate dihydrate in 500ml of dimethylformamide (DMF), stir and dissolve 5.98g of terephthalic acid (BDC) in 400ml of DMF and add 8.52ml of triethylamine, mix the two solutions Stir magnetically at 20°C for 8 hours, obtain the precipitate by centrifugation, soak and wash with DMF, dichloromethane, and DMF in sequence, and soak for 6 hours each time, and finally pre-dry the product at 60°C, and then vacuum at 150°C After drying for 18 hours, the Zn-based metal-organic framework was obtained. The product was calcined for 2 hours under nitrogen atmosphere, the calcining temperature was 600° C., and the heating rate was 5° C. / min.
[0050] Weigh 0.1g calcined zinc sulfide / porous carbon composite material and 0.2g thiourea and disperse in 40ml ethylene glycol, transfer the solution to a 60ml reactor, seal it and react at 180°C for 18h. After the reaction, the reactor was cooled in the air, and the cooled reaction solution was suction-filtere...
Embodiment 3
[0052] Stir and dissolve 19.75g of zinc acetate dihydrate in 500ml of dimethylformamide (DMF), stir and dissolve 5.98g of terephthalic acid (BDC) in 400ml of DMF and add 8.52ml of triethylamine, mix the two solutions Stir magnetically at 20°C for 6h, obtain the precipitate by centrifugation, soak and wash with DMF, dichloromethane, and DMF in turn, and soak for 6h each time, and finally pre-dry the product at 60°C, and then vacuum at 150°C Dry for 12h. The dried MOF-5 was calcined for 2 hours under nitrogen atmosphere, the calcination temperature was 650°C, and the heating rate was 5°C / min.
[0053] Weigh 0.1g calcined zinc sulfide / porous carbon composite material and 0.4g sodium sulfide nonahydrate and disperse in a mixed solution of 20ml ethylenediamine and 20ml deionized water, transfer the mixed solution to a 60ml reactor and seal it at 120 Reaction at ℃ for 24h. After the reaction, the reactor was cooled in the air, and the cooled reaction solution was suction-filtered,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| First discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com