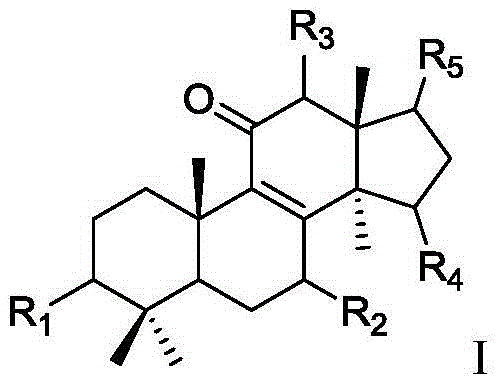
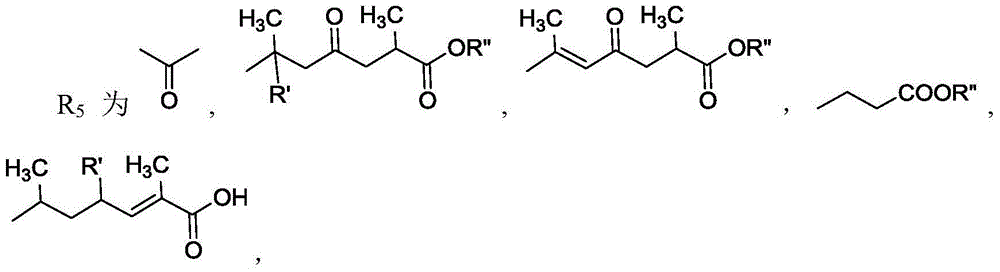
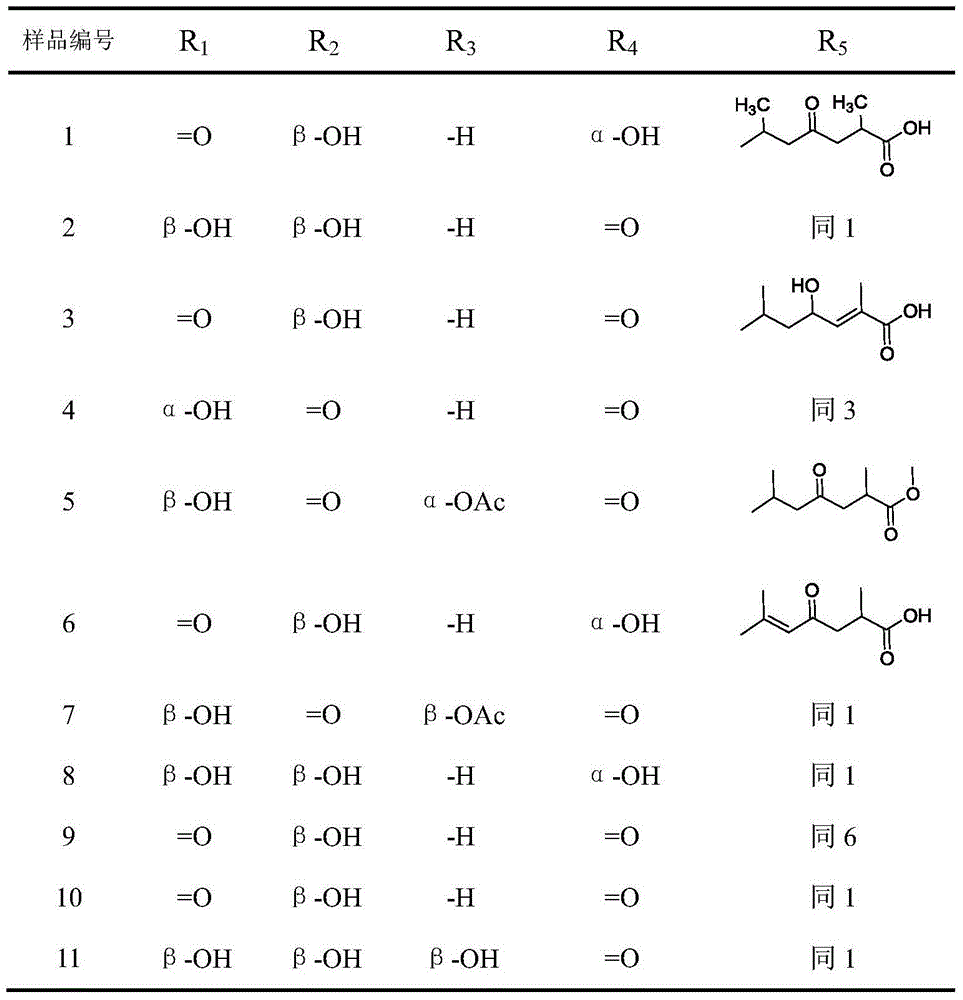
11 beta-hydroxysteroid dehydrogenase inhibitor and its pharmaceutical composition and use
A technology of use, medicine, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 11β-HSD inhibitory activity test
[0028] 1. Experimental materials
[0029] Reagents: Ganoderma lucidum triterpene acid, all self-made by our research group, with a purity greater than 95%; cortisone, hydrocortisone, prednisone acetate and nicotinamide adenine dinucleotide phosphate (NADPH) were purchased from Bailingwei Reagent Company; HPLC uses methanol as chromatographically pure, and the rest of the reagents are analytical grade domestic reagents.
[0030] Animals: Wistar rats, weighing 240-300 g, were bred and raised by the animal room of Tianjin Institute of Pharmaceutical Research.
[0031] Instruments: 515 type high performance liquid chromatography pump (Waters Company), 717plus autosampler (Waters Company), 2487 type ultraviolet detector (Waters Company).
[0032]2. Experimental method:
[0033] 2.1 Enzyme activity inhibition experiment
[0034] Preparation of liver microsomes and analysis of 11β-HSD1 activity: several rats were taken, anesthetized with ...
Embodiment 2
[0046] Grind and dry 6 kg of Chizhi fruiting body, heat and reflux extract with 70% ethanol for 3 times, each time for 2 hours, combine the extracts and concentrate under reduced pressure until there is no alcohol smell, disperse with a small amount of water, add 3 times the amount of acetone to stir, let stand, and filter , and the filtrate was concentrated under reduced pressure to obtain 120 g of liquid extract. Transfer to methanol, mix the sample with 60-100 mesh silica gel, carry out silica gel column chromatography (1200g), and use dichloromethane alcohol-methanol system (100:0~3:1) gradient elution, and collect and wash each 500ml. The liquid was removed, and the same components were combined for TLC detection to obtain 21 components.
[0047] Components 10 and 11 were concentrated to obtain 19.4g. Weighed 19.4g of sample mixing silica gel (60-100 mesh), dissolved the sample in methanol and mixed the sample, and weighed the silicon dichloromethane-methanol system (100:...
Embodiment 3
[0055] Take 10 g of compound No. 8, add 30 g of lactose-microcrystalline cellulose (4:1), 1% magnesium stearate, granulate with 70% ethanol, and compress into tablets to obtain 1000 tablets. Specification 10mg / tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com