Stable ambroxol hydrochloride huge capacity injection and preparation method thereof
A technology of ambroxol hydrochloride and injection, applied in the field of medical injection, can solve problems such as inconvenience of use, increased pollution opportunities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A stable large volume injection of ambroxol hydrochloride, comprising the following substances: 0.15 g of ambroxol hydrochloride, 25 g of fructose, 1.8 g of gallic acid, 6.5 g of citric acid, 13.8 g of disodium hydrogen phosphate, and adding water for injection to 500 ml.
[0033] A kind of stable ambroxol hydrochloride large volume injection, comprises the following steps:
[0034] (1) Concentrated formulation: add water for injection of 30% of the prescription amount into the concentrated formulation tank, and control the temperature at 50-60°C; add the fructose of the prescription amount, stir to dissolve it, add 0.1% (g / ml) Wet medicinal charcoal, stir for 30 minutes, filter for decarbonization, filter into the dilute preparation tank, rinse the concentrated preparation tank with water for injection several times, and filter into the dilute preparation tank;
[0035] (2) Dilute formulation: add water for injection to 80% of the prescription volume in the dilute form...
Embodiment 2
[0039] A stable ambroxol hydrochloride large-volume injection comprises the following substances: 0.30 g of ambroxol hydrochloride, 50 g of fructose, 3.6 g of gallic acid, 18 g of sodium acetate, and 9.8 ml of glacial acetic acid. Water for injection 1000mL.
[0040] A kind of stable ambroxol hydrochloride large volume injection, comprises the following steps:
[0041] (1) Concentrated formulation: Add water for injection of 50% of the prescription amount into the concentrated formulation tank, and control the temperature at 70-80°C; add the fructose of the prescription amount, stir to dissolve it, add 0.1% (g / ml) Wet medicinal charcoal, stir for 30 minutes, filter for decarbonization, filter into the dilute preparation tank, rinse the concentrated preparation tank with water for injection several times, and filter into the dilute preparation tank;
[0042] (2) Dilute formulation: add water for injection up to 90% of the prescription volume to the dilute formulation tank, adj...
Embodiment 3
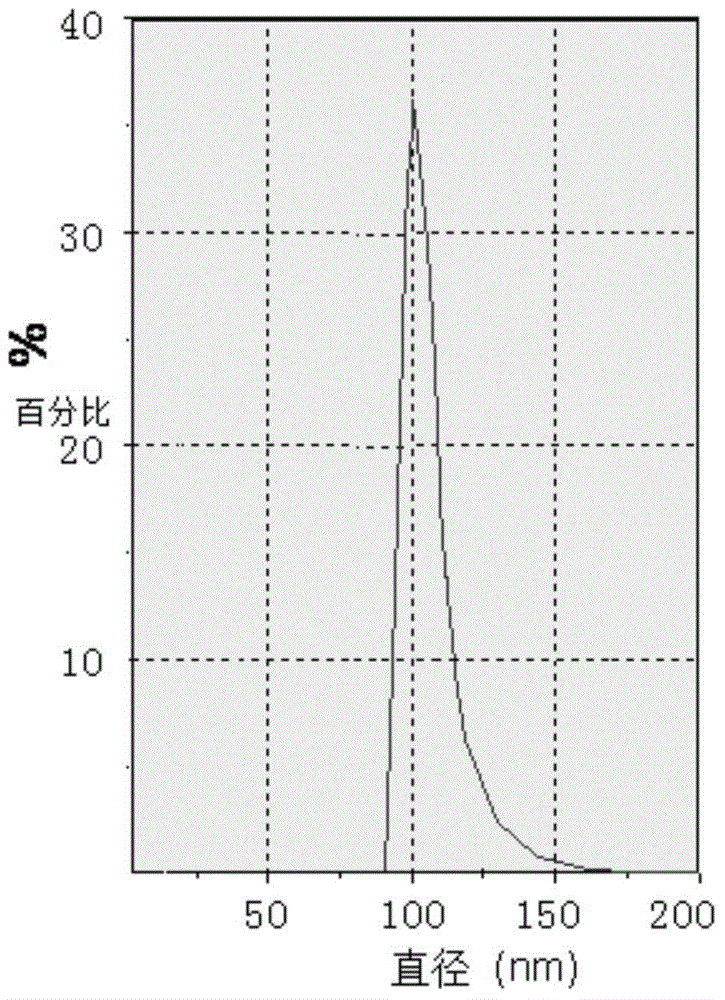
[0046] A kind of stable ambroxol hydrochloride large-capacity injection comprises the following materials: ambroxol hydrochloride nanoparticles (containing ambroxol hydrochloride 150mg), fructose 25g, gallic acid 1.8g, citric acid 6.5, disodium hydrogen phosphate 13.8 g, 500 mL of water for injection.
[0047] The preparation method of described ambroxol hydrochloride nanoparticles is:
[0048] A, medicine ambroxol hydrochloride 0.15g and macromolecule auxiliary material polylactic acid 1.0g are dissolved respectively and become drug solution and macromolecule auxiliary material solution;
[0049] B, mixing the drug solution and the polymer auxiliary material solution; the weight ratio of the drug and the polymer auxiliary material is 15-85:100;
[0050] C. The mixed solution is ultrasonically emulsified, and the emulsification time is 5-60 minutes to obtain an emulsion;
[0051] D. Stir the emulsion at 200-1000 rpm for 0.5-2 hours to obtain coagulation;
[0052] E. Add glu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com