Method of removing nitrate nitrogen in wastewater
A technology for nitrate nitrogen and wastewater, applied in chemical instruments and methods, multi-stage water/sewage treatment, water/sludge/sewage treatment, etc., can solve the problems of complex catalyst preparation and high price, and achieve enhanced reduction efficiency, Effect of reduction efficiency improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
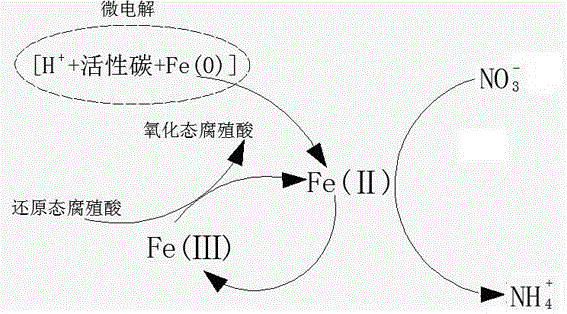
[0030] A method for removing nitrate nitrogen in waste water, it comprises the following steps:
[0031] S1. Adjust the pH value: collect the alkaline wastewater into an acid and alkali resistant container, and use sulfuric acid to adjust the pH value of the wastewater to 2;
[0032] S2. Denitrification: add humic acid, activated carbon and iron filings to the wastewater to adjust the pH value, the mass ratio of humic acid, iron filings and activated carbon is 1:2:1, the mass of nitrate nitrogen and iron filings in the wastewater The ratio is 1:20, under the conditions of isolation from the outside air and stirring at 80r / min, react for 60 minutes, separate solid and liquid, and collect the liquid into the chemical sedimentation tank;
[0033] S3. Chemical precipitation: Add disodium hydrogen phosphate dodecahydrate and magnesium chloride hexahydrate to the chemical precipitation tank under the condition of aeration and stirring, and the aeration volume of aeration is 0.1 m 3...
Embodiment 2
[0035] A method for removing nitrate nitrogen in waste water, it comprises the following steps:
[0036] S1. Adjust the pH value: collect the alkaline wastewater into an acid and alkali resistant container, and use sulfuric acid to adjust the pH value of the wastewater to 4;
[0037] S2. Denitrification: add humic acid, activated carbon and iron filings to the wastewater to adjust the pH value, the mass ratio of humic acid, iron filings and activated carbon is 1:100:100, the mass of nitrate nitrogen and iron filings in the wastewater The ratio is 1:350, and the reaction is stirred for 180 minutes under the condition of being isolated from the outside air and the intensity is 200r / min, the solid and liquid are separated, and the liquid is collected into the chemical sedimentation tank;
[0038] S3. Chemical precipitation: Add disodium hydrogen phosphate dodecahydrate and magnesium chloride hexahydrate to the chemical precipitation tank under the condition of aeration and stirri...
Embodiment 3
[0040] A method for removing nitrate nitrogen in waste water, it comprises the following steps:
[0041] S1. Adjust the pH value: collect the alkaline wastewater into an acid and alkali resistant container, and use sulfuric acid to adjust the pH value of the wastewater to 3;
[0042] S2. Denitrification: add humic acid, activated carbon and iron filings to the wastewater to adjust the pH value, the mass ratio of humic acid, iron filings and activated carbon is 1:30:20, the mass of nitrate nitrogen and iron filings in the wastewater The ratio is 1:100, and the reaction is stirred for 100 minutes under the conditions of isolation from the outside air and the intensity of 120r / min, the separation of solid and liquid, and the liquid is collected into the chemical sedimentation tank;
[0043] S3. Chemical precipitation: Add disodium hydrogen phosphate dodecahydrate and magnesium chloride hexahydrate to the chemical precipitation tank under the condition of aeration and stirring, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com