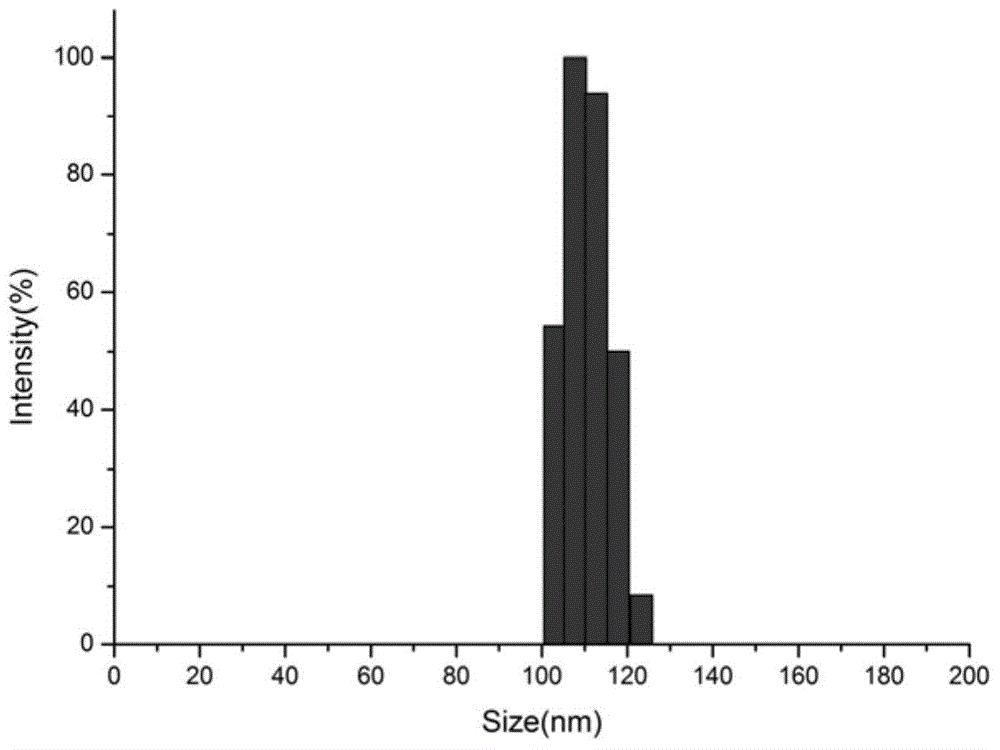
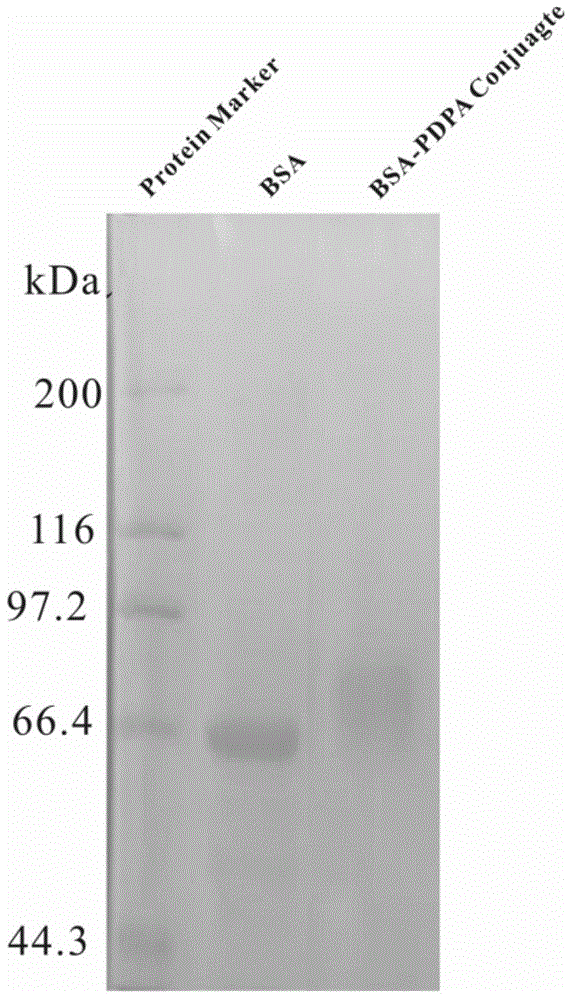
ph responsiveness protein-macromolecule combination and preparing and assembling method thereof
A responsive, high-molecular technology, applied in the direction of non-active components of high-molecular compounds, can solve the problems that are rarely transformed into clinical and put into the market, and the controllability of the size and shape of nanocarriers limits the development, etc., to achieve a good pH Responsiveness, low toxicity, and high production yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Synthesis of Bovine Serum Albumin (BSA) Macromolecular ATRP Initiator.
[0054] ① Disperse 30g of maleic anhydride in 150ml of toluene, stir and heat in an oil bath to 80°C, slowly inject 33.3ml of furan with a syringe, condense and reflux for 6 hours. After the reaction was completed, it was lowered to room temperature, allowed to stand for 1 h, filtered under reduced pressure, washed 3 times with petroleum ether, and a white product A was obtained.
[0055] ②Take 10g of product A, disperse it in 100ml of methanol, stir and cool in an ice bath to 0°C, slowly add 3.6ml of ethanolamine dropwise to the above solution, stir at 0°C for 20min, then stir at room temperature for 35min, and reflux in a stirring oil bath at 80°C 24h. After the reaction, cooling and recrystallization, filtration under reduced pressure, white crystal B was obtained.
[0056] ③ Take 3g of product B and 2.16ml of triethylamine, add it to 170ml of THF at 0°C, dissolve 1.89ml of 2-bromoisobutyryl br...
Embodiment 2
[0061] Synthesis of Bovine Serum Albumin (BSA) Macromolecular ATRP Initiator.
[0062] ① Disperse 40g of maleic anhydride in 180ml of toluene, stir and heat in an oil bath to 90°C, slowly inject 44.4ml of furan with a syringe, condense and reflux for 8 hours. After the reaction was completed, it was lowered to room temperature, allowed to stand for 2 hours, filtered under reduced pressure, washed twice with petroleum ether, and a white product A was obtained.
[0063] ②Take 20g of product A, disperse it in 80ml of methanol, stir and cool in an ice bath to 0°C, slowly add 7.2ml of ethanolamine to the above solution dropwise, stir at 0°C for 15min, then stir at room temperature for 40min, and reflux in a stirring oil bath at 90°C 36h. After the reaction, cooling and recrystallization, filtration under reduced pressure, white crystal B was obtained.
[0064] ③ Take 6g of product B and 4.32ml of triethylamine, add it to 170ml of THF at 0°C, dissolve 3.78ml of 2-bromoisobutyryl b...
Embodiment 3
[0068] Synthesis of Bovine Serum Albumin (BSA) Macromolecular ATRP Initiator.
[0069] ① Disperse 20g of maleic anhydride in 120ml of toluene, stir and heat in an oil bath to 100°C, slowly inject 22.2ml of furan with a syringe, condense and reflux for 7 hours. After the reaction was completed, it was lowered to room temperature, allowed to stand for 3 hours, filtered under reduced pressure, washed 3 times with petroleum ether, and a white product A was obtained.
[0070] ②Take 15g of product A, disperse it in 90ml of methanol, stir and cool in an ice bath to 0°C, slowly add 5.4ml of ethanolamine dropwise to the above solution, stir at 0°C for 25min, then stir at room temperature for 30min, and reflux in a stirring oil bath at 100°C 48h. After the reaction, cooling and recrystallization, filtration under reduced pressure, white crystal B was obtained.
[0071] ③Take 4g of product B and 2.88ml of triethylamine, add it to 150ml THF at 0°C, dissolve 2.52ml of 2-bromoisobutyryl b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Effective particle size | aaaaa | aaaaa |
| Effective particle size | aaaaa | aaaaa |
| Effective particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com