Preparation method of hydroxy benzene anhydride
A technology of hydroxyphthalic anhydride and hydroxyphthalonitrile, which is applied in the field of simple preparation of hydroxyphthalic anhydride, can solve the problems of inconvenient industrial production, harsh equipment requirements, unsuitable reactor materials, etc., and achieves simple operation, mild reaction conditions, and easy control. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
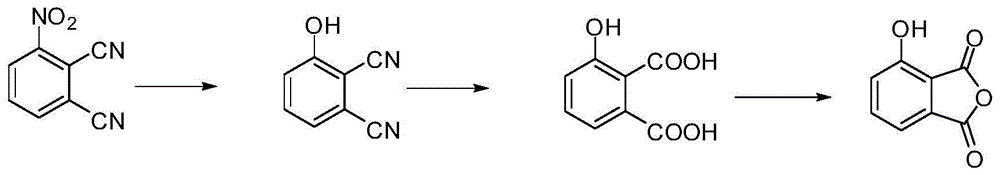
[0034] Embodiment 1: the preparation of 3-hydroxyphthalic anhydride:
[0035] (1) Add 103.9g (600mmol) 3-nitrophthalonitrile and 800mL dimethyl sulfoxide to the three-necked flask in sequence. After the powder is dissolved, add 85.7g (620mmol) potassium carbonate and 41.4g (600mmol) nitrous acid Sodium, heated to reflux at 160°C, reacted for 3 hours, cooled to room temperature, poured into a large amount of water, adjusted pH=3 with concentrated HCl, precipitated a yellow solid, vacuum-dried at 110°C after suction filtration, and obtained 76.4 g of 3-hydroxyphthalonitrile, Yield: 88%.
[0036] (2) Add 209.8g (3.74mol) of potassium hydroxide and 800mL of water into a three-necked flask. After exothermic dissolution, add 69.2g (480mmol) of 3-hydroxyphthalonitrile, reflux at 130°C for 90 hours, filter, and collect the filtrate , after cooling, acidify HCl to pH = 1, after cooling, add ethyl acetate to extract 3 times, take the upper ethyl acetate layer, add anhydrous magnesium s...
Embodiment 2
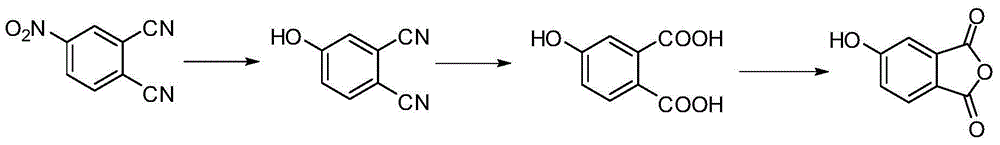
[0041] Embodiment 2: the preparation of 4-hydroxyphthalic anhydride:
[0042] (1) Add 103.9g (600mmol) 4-nitrophthalonitrile and 800mL dimethyl sulfoxide to the three-necked flask in turn. After the powder is dissolved, add 91.0g (660mmol) potassium carbonate and 46.0g (660mmol) nitrous acid Sodium, heated to reflux at 160°C, reacted for 5 hours, cooled to room temperature, poured into a large amount of water, adjusted the pH to 3 with concentrated HCl, and precipitated a yellow solid, after suction filtration, vacuum-dried at 110°C to obtain 73.3g of 4-hydroxyphthalonitrile , Yield: 85%.
[0043] (2) Add 230.0g (4.11mol) of potassium hydroxide and 800mL of water into a three-necked flask. After exothermic dissolution, add 69.2g (480mmol) of 4-hydroxyphthalonitrile, reflux at 100°C for 90 hours, filter, and collect the filtrate , after cooling, acidify with HCl to PH = 1, after cooling, extract with ethyl acetate 3 times, take the upper layer of ethyl acetate, add anhydrous m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com