Dual-network poly(N-acryloyl-L-alpha-amino acid)/hyaluronic acid composite hydrogel and preparation method thereof
A composite hydrogel and acryloyl technology, applied in the field of biomedical materials, can solve the problems of few applications, hydrogel brittleness and large modulus, and achieve the effect of simple process, good mechanical properties, and rapid reduction of dissociation properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Preparation of Hydrazide Hyaluronic Acid
[0029] Carbodiimide method is used to graft 2,2'-dithiodiacetylhydrazide on hyaluronic acid with a molecular weight of 1 million to obtain hydrazide hyaluronic acid with a substitution degree of 40%. The details are as follows: mix hyaluronic acid, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride with N-hydroxysuccinimide, 2,2'-dithiodiacetyl Hydrazine was dissolved in distilled water to prepare a hyaluronic acid solution with a concentration of 1%. The pH value of the system was adjusted to 5 with 1mol / L hydrochloric acid, and the reaction was stirred at room temperature for 48 hours. After the reaction, the reaction system was dialyzed with deionized water for 2 days ( The molecular weight cut-off of the dialysis bag is 3500Da, the same below) and freeze-dried at minus 20°C for 72 hours to obtain hydrazide hyaluronic acid. Among them, the number of repeating units of hyaluronic acid, 1-(3-dimethylaminopropyl)-3...
Embodiment 2
[0040] 1) Preparation of Hydrazide Hyaluronic Acid
[0041] Carbodiimide method is used to graft 2,2'-dithiodiacetylhydrazide on hyaluronic acid with a molecular weight of 2 million to obtain hydrazide hyaluronic acid with a substitution degree of 30%. The details are as follows: mix hyaluronic acid, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride with N-hydroxysuccinimide, 2,2'-dithiodiacetyl Hydrazine was dissolved in distilled water to prepare a hyaluronic acid solution with a concentration of 0.5%. The pH value of the system was adjusted to 5.5 with 1mol / L hydrochloric acid, and the reaction was stirred at room temperature for 24 hours. Dialyzed with distilled water for 2 days with a molecular weight cut-off of 3500Da, the Freeze drying at minus 20°C for 72 hours to obtain hydrazide hyaluronic acid. Among them, the number of repeating units of hyaluronic acid, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, N-hydroxysuccinimide, 2,2'-dithiobis Th...
Embodiment 3
[0051] 1) Preparation of Hydrazide Hyaluronic Acid
[0052] Using the carbodiimide method, 3,3'-dithiodipropionyl hydrazide was grafted onto hyaluronic acid with a molecular weight of 800,000 to obtain hydrazide hyaluronic acid with a substitution degree of 30%. The details are as follows: mix hyaluronic acid, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride with N-hydroxysuccinimide, 3,3'-dithiodipropyl Hydrazide was dissolved in distilled water to prepare a hyaluronic acid solution with a concentration of 0.8%. The pH value of the system was adjusted to 5 with 1mol / L hydrochloric acid. The reaction was stirred at room temperature for 24 hours, and the distilled water was dialyzed for 2 hours with a dialysis bag with a molecular weight cut-off of 3500Da. Two days later, it was freeze-dried at minus 20°C for 72 hours to obtain hydrazidated hyaluronic acid. Among them, the number of repeating units of hyaluronic acid, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
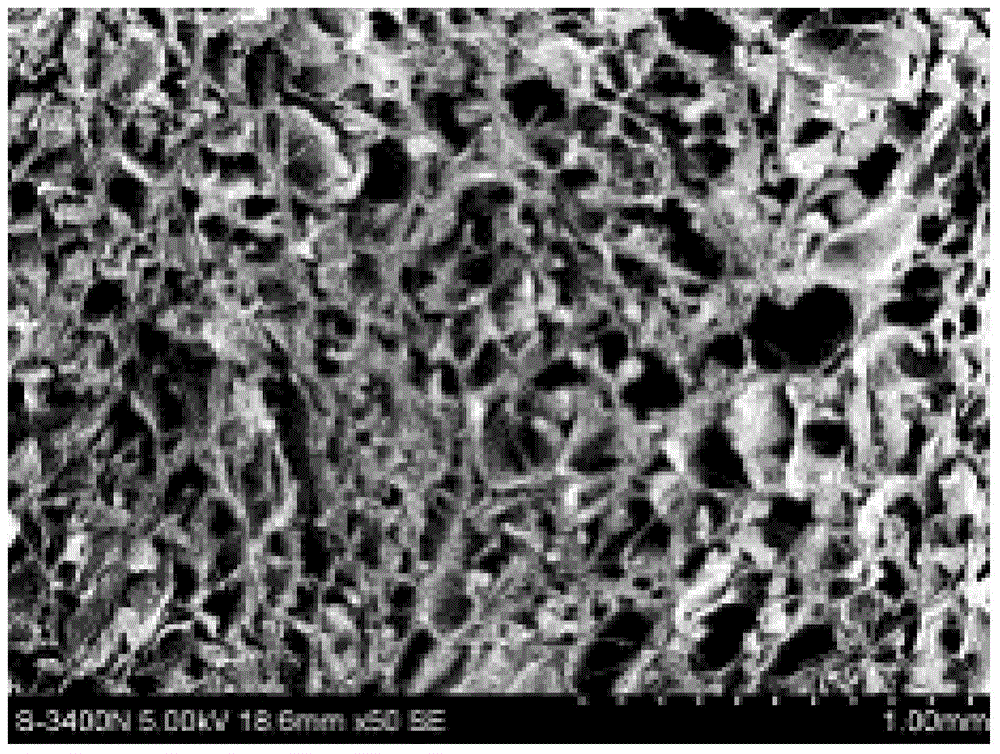
| Pore size distribution range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com