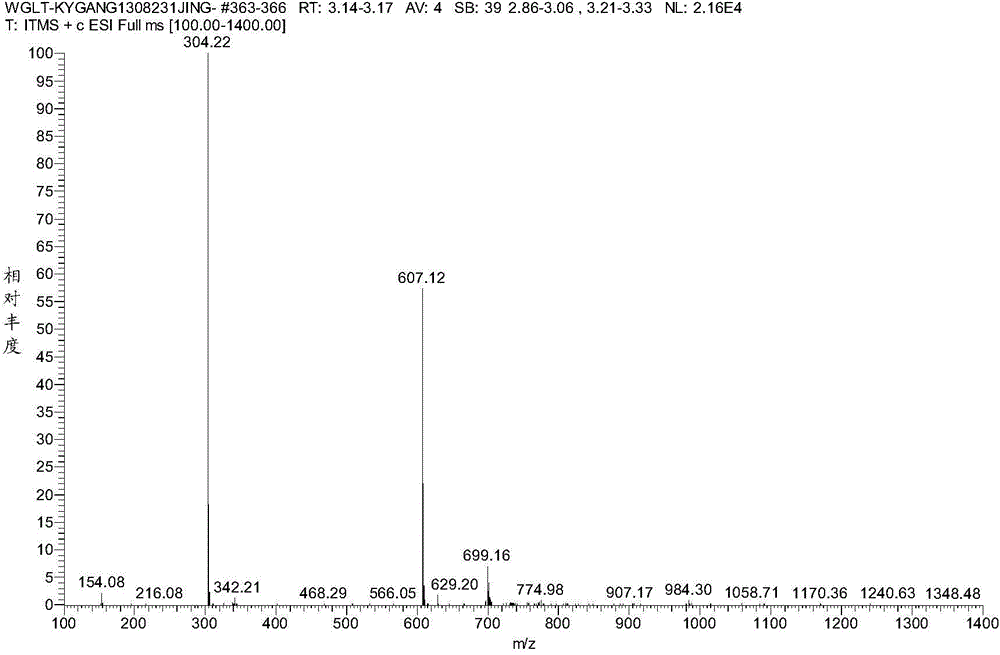
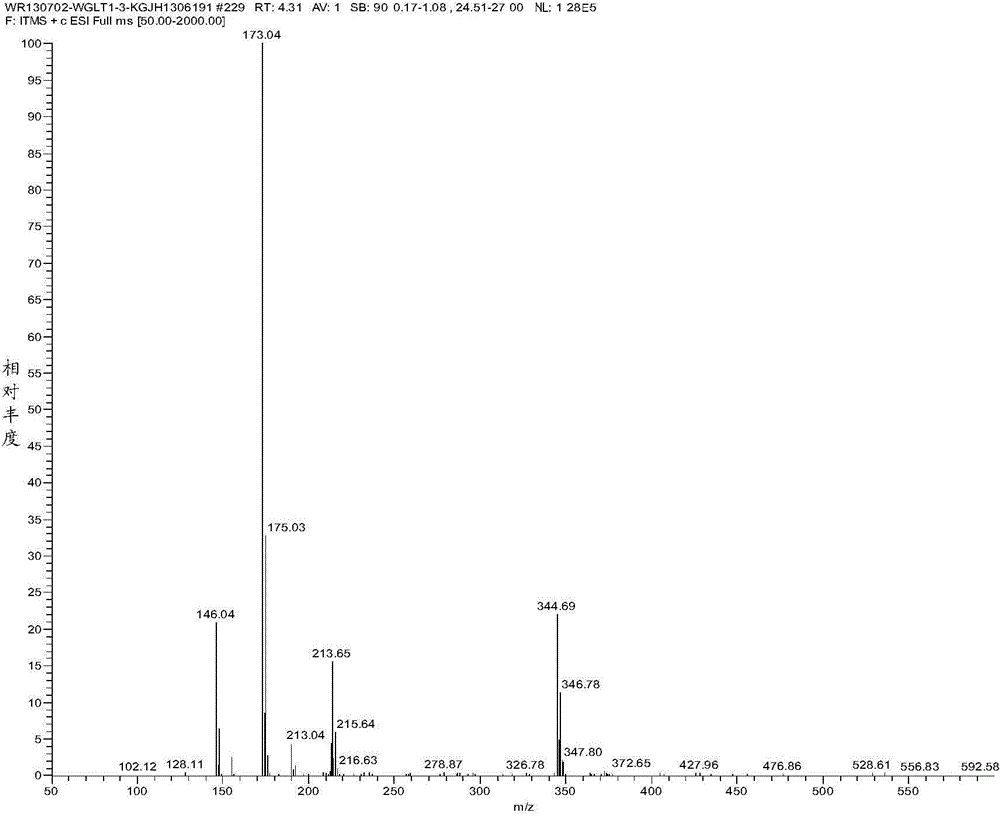
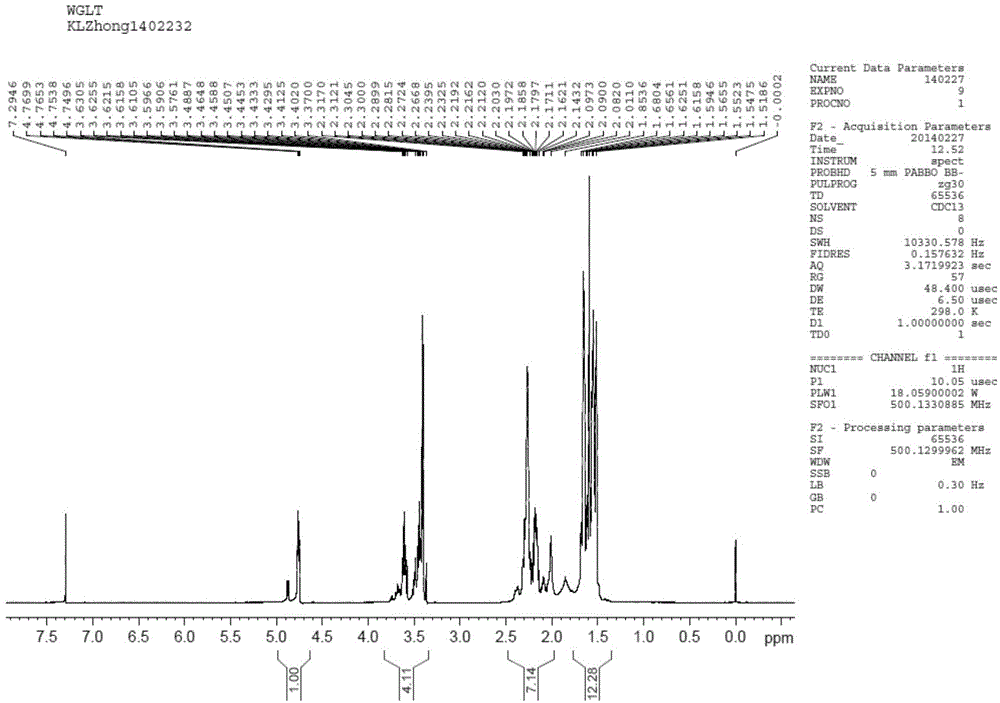
Efficient synthesis method of vildagliptin
A synthetic method and high-efficiency technology, applied in the direction of organic chemistry, etc., can solve the problems of main product purity and yield reduction, poor acid application effect, and increased production, so as to avoid adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of (S)-1-(2-chloroacetyl chloride)pyrrolidine-2-carbonitrile
[0040] In a 2L three-neck flask, add 100.0g (0.8772moL) L-prolinamide, 640g dichloromethane and 102.4g (1.0139moL) triethylamine in sequence to form a suspension, stir and cool down to -25°C, Add 116.0g (1.0265moL) chloroacetyl chloride dropwise into the solution, and control the dropping temperature ≤ -15°C; after the dropwise addition, stir and react at -25--15°C for 1h, take the sample and dissolve it in methanol, and TLC detects that the reaction is complete, and (S )-1-(2-chloroacetyl chloride) pyrrolidine-2-carboxamide suspension;
[0041](S)-1-(2-Chloroacetyl chloride)pyrrolidine-2-carboxamide suspension was warmed up to -5-5°C, without separation, 147.2g (2.0164moL) DMF ( N,N-Dimethylformamide) and 150.8g (0.9837moL) of phosphorus oxychloride, the dropwise addition was completed, and the temperature was kept at -5-5°C for 2 hours, the sample was dissolved and diluted with methanol, and t...
Embodiment 2
[0045] Preparation of (S)-1-(2-chloroacetyl chloride)pyrrolidine-2-carbonitrile
[0046] In a 2L three-neck flask, add 100.0g (0.8772moL) L-prolinamide, 640g ethyl acetate and 102.4g (1.0139moL) triethylamine in sequence, stir and cool down to -35°C, add dropwise 116.0g (1.0265moL) Chloroacetyl chloride, control the dropping temperature ≤ -25°C; after the dropwise addition, stir and react at -35--25°C for 1h, take the sample and dissolve it in methanol, TLC detects that the reaction is complete, and (S)-1-(2-chloroacetyl chloride is obtained ) pyrrolidine-2-carboxamide suspension;
[0047] (S)-1-(2-Chloroacetyl chloride)pyrrolidine-2-carboxamide suspension was warmed up to 10-20°C, without separation, 147.2g (2.0164moL) DMF and 150.8 g (0.9837moL) phosphorus oxychloride, after the dropwise addition, keep warm at 10-20°C for 2 hours, dissolve and dilute the sample with methanol, and TLC detects that the reaction is complete; add 700.0g of water to quench the reaction, separate...
Embodiment 3
[0051] Preparation of (S)-1-(2-chloroacetyl chloride)pyrrolidine-2-carbonitrile
[0052] In a 2L three-neck flask, add 100.0g (0.8772moL) L-prolinamide, 640g dichloromethane and 102.4g (1.0139moL) triethylamine in sequence to form a suspension, stir and cool down to -25°C, Add 116.0g (1.0265moL) chloroacetyl chloride dropwise into the solution, and control the dropping temperature ≤ -15°C; after the dropwise addition, stir and react at -25--15°C for 1h, take the sample and dissolve it in methanol, and TLC detects that the reaction is complete, and (S )-1-(2-chloroacetyl chloride) pyrrolidine-2-carboxamide suspension;
[0053] (S)-1-(2-Chloroacetyl chloride)pyrrolidine-2-carboxamide suspension was warmed up to -5-5°C, without separation, 147.2g (2.0164moL) DMF ( N,N-Dimethylformamide) and 150.8g (0.9837moL) of phosphorus oxychloride, the dropwise addition was completed, and the temperature was kept at -5-5°C for 2 hours, the sample was dissolved and diluted with methanol, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com