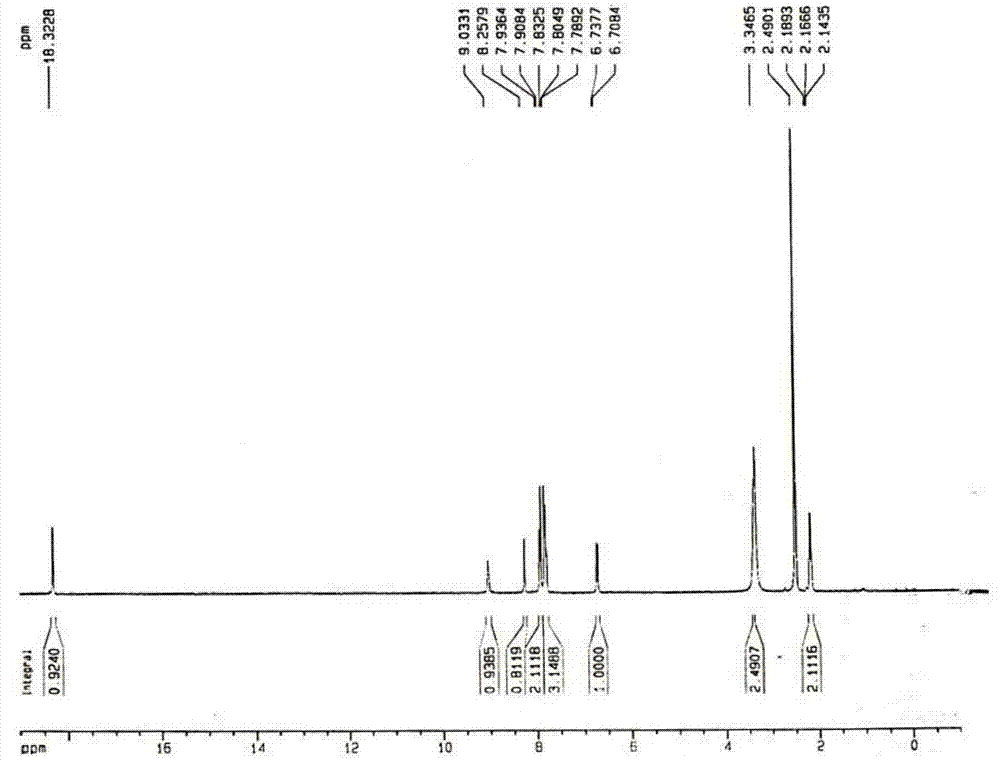
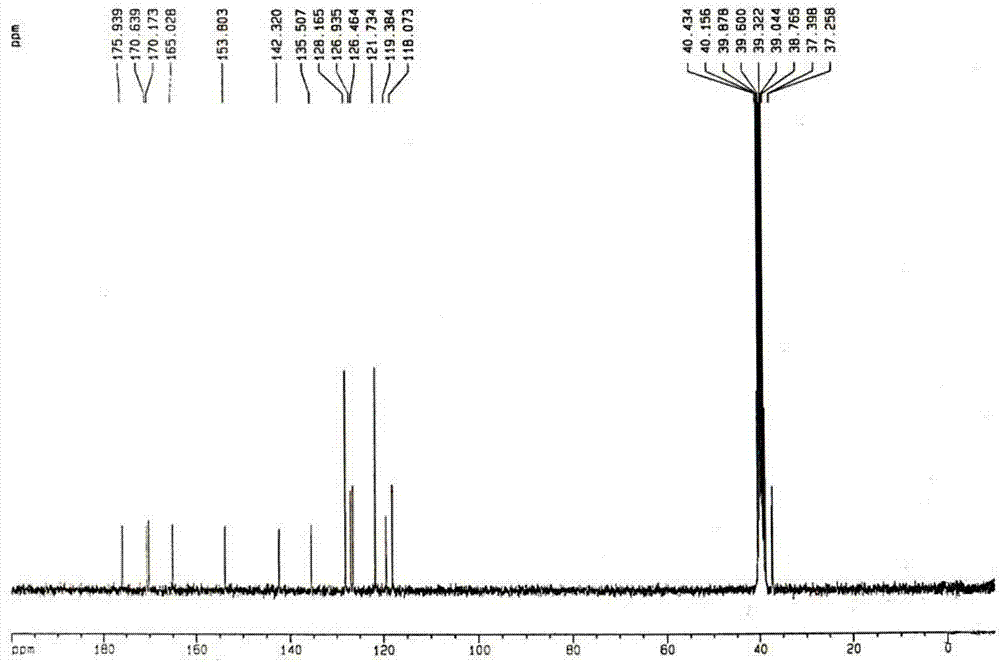
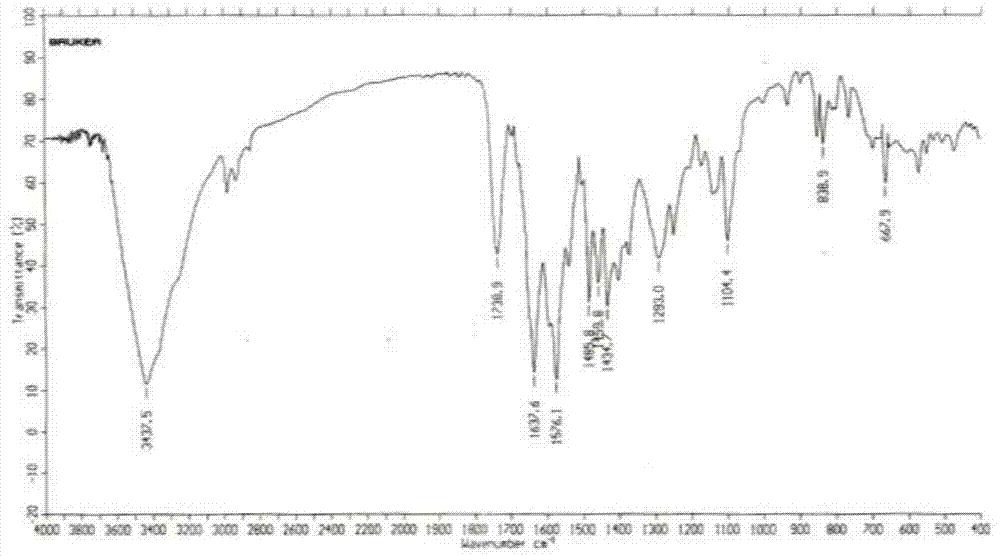
A kind of preparation method of balsalazide sodium
A technology of balsalazide sodium and balsalazide, which is applied in the field of medicine, can solve the problems of reducing operational risk, high adsorption capacity of activated carbon, and increasing production costs, and achieves reduced production costs and environmental pollution, mild reaction conditions, and improved production efficiency. safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation of embodiment 1, balsalazide sodium
[0047] (1) Dissolve 50g of β-alanine in 500ml of sodium hydroxide aqueous solution, then add 100g of p-nitrobenzoyl chloride, and react for 2 hours under stirring, then filter, wash with water, and vacuum dry the dried product with acetone Recrystallized to obtain p-nitrobenzoyl-β-alanine with a yield of 84.3%, mp: 163-164°C;
[0048] (2) Add 40g of the p-nitrobenzoyl-β-alanine obtained in step 1) and 100ml of solvent water to the reactor, dissolve and add the catalyst FeCl at room temperature 3 ·6H 2 O reacted with reducing agent hydrazine hydrate for 2 hours, in which the catalyst FeCl 3 ·6H 2 The amount of O is 2.5% of the mass of p-nitrobenzoyl-β-alanine, and the molar ratio of p-nitrobenzoyl-β-alanine to hydrazine hydrate is 1:2.5; then adjust the pH value with hydrochloric acid to 1-2, filtered, washed with water, and dried to obtain p-aminobenzoyl-β-alanine with a yield of 90.4%, mp: 154-156°C, and a purit...
Embodiment 2
[0054] The preparation of embodiment 2, balsalazide sodium
[0055] (1) Dissolve 90g of β-alanine in 500ml of sodium hydroxide aqueous solution, then add 150g of p-nitrobenzoyl chloride; react for 4 hours under stirring, then filter, wash with water, and dry in vacuum. The dried product is washed with acetone Recrystallized to obtain p-nitrobenzoyl-β-alanine with a yield of 82.5%, mp: 163-164°C;
[0056] (2) Add 40g of the p-nitrobenzoyl-β-alanine obtained in step 1) and 100ml of solvent water to the reactor, dissolve and add the catalyst FeCl at room temperature 3 ·6H 2 O reacted with reducing agent hydrazine hydrate for 1 hour, in which the catalyst FeCl 3 ·6H 2 The amount of O is 2.0% of the mass of p-nitrobenzoyl-β-alanine, and the molar ratio of p-nitrobenzoyl-β-alanine to hydrazine hydrate is 1:1.5; then adjust the pH value with hydrochloric acid to 1-2, filtered, washed with water, and dried to obtain p-aminobenzoyl-β-alanine with a yield of 89.9%, mp: 154-156°C, an...
Embodiment 3
[0060] Embodiment 3, the preparation of balsalazide sodium
[0061] (1) Dissolve 60g of β-alanine in 500ml of sodium hydroxide aqueous solution, then add 120g of p-nitrobenzoyl chloride; stir for 3 hours, then filter, wash with water, and vacuum dry the dried product with acetone Recrystallized to obtain p-nitrobenzoyl-β-alanine with a yield of 80.1%;
[0062] (2) Add 40g of the p-nitrobenzoyl-β-alanine obtained in step 1) and 100ml of solvent water to the reactor, dissolve and add the catalyst FeCl at room temperature 3 ·6H 2 O reacted with reducing agent hydrazine hydrate for 2.5 hours, wherein the catalyst FeCl 3 ·6H 2 The amount of O is 3.0% of the mass of p-nitrobenzoyl-β-alanine, and the molar ratio of p-nitrobenzoyl-β-alanine to hydrazine hydrate is 1:3; then adjust the pH value with hydrochloric acid to 1-2, filtered, washed with water, and dried to obtain p-aminobenzoyl-β-alanine with a yield of 89.9%, mp: 154-156°C, and a purity (HPLC) greater than 98.0%;
[006...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com