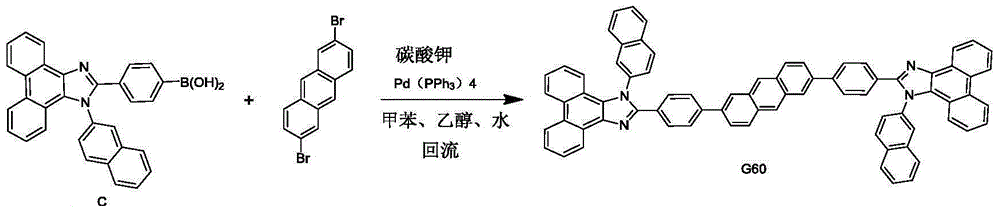
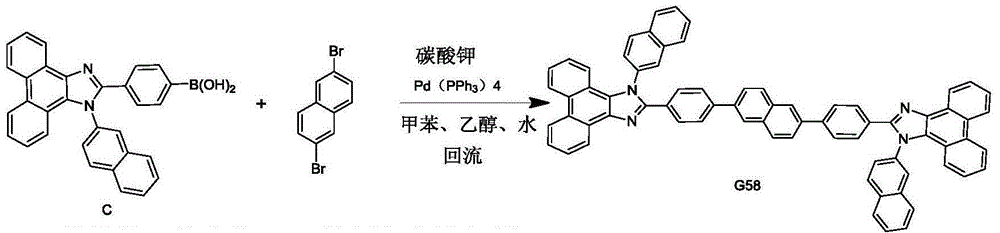
Phenanthroimidazole symmetric derivative host material and electroluminescent device
A technology of phenanthroimidazole and host material, which is applied in the field of phenanthroimidazole symmetrical derivative host material and electroluminescent device, and can solve the problems of unsatisfactory luminous efficiency, lifetime and optical purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1: the preparation of compound G1
[0089] The first step: prepare intermediate A according to the following steps:
[0090]
[0091] (1) Synthesis of intermediate A-1:
[0092] Add 2.08g (0.01mol) phenanthrene-9,10 diketone, 2.04g (0.011mol) p-bromobenzaldehyde, 0.186g (0.02mol) aniline, 50ml acetic acid in a 100ml three-necked flask, add ammonium acetate dropwise under nitrogen protection After the addition was completed, the temperature was lowered to 25°C after 130°C reaction for 18 hours. The reaction solution was filtered, washed with water and saturated sodium bicarbonate to obtain 3.4 g of the product, hplc: 98%, yield: 76%.
[0093] (2) Synthesis of Intermediate A:
[0094] Add 9g of 2-(4-bromophenyl)-1-phenylphenanthroimidazole (0.02mol) into 150ml THF, cool down to -78°C, and drop 12ml (2.5M / L, 0.03mol ) n-BuLi, keep warm for 30 minutes, add 4.26g (0.041mol) trimethyl borate dropwise, finish the reaction after 2 hours of reaction, add 50ml o...
Embodiment 2
[0100] Embodiment 2: the preparation of compound G3
[0101] The preparation of the target compound G3 of this embodiment, its structural formula and synthetic route are as follows:
[0102]
[0103] Specifically, the preparation method of compound G3 includes:
[0104] (1) Intermediate A is prepared according to the same method as in Example 1;
[0105] (2) 8.28g (0.02mol) of intermediate A, 2.86g (0.01mol) of 1,4-dibromonaphthalene, 4.15g (0.03mol) of potassium carbonate, 50ml of toluene, 30ml of ethanol and 30ml of water were mixed, and the Next, add tetrakis(triphenylphosphine) palladium 0.23g (0.0002mol) to react, raise the temperature to reflux, point the plate to monitor until the reaction is complete, end the reaction, spin the reaction solution to dryness, add 100ml of dichloromethane, make it dissolve completely and Pass through a silica gel column, add 100ml of water to the filtrate, wash with water, separate the organic phase, spin it to dryness, boil it twice...
Embodiment 3
[0106] Embodiment 3: the preparation of compound G4
[0107] The preparation of the target compound G4 of this embodiment, its structural formula and synthetic route are as follows:
[0108]
[0109] Specifically, the preparation method of the compound G4 includes:
[0110] (1) Intermediate A is prepared according to the same method as in Example 1;
[0111] (2) 8.28g (0.02mol) of intermediate A, 2.86g (0.01mol) of 1,5-dibromonaphthalene, 4.15g (0.03mol) of potassium carbonate, 50ml of toluene, 30ml of ethanol and 30ml of water were mixed, and the Next, add tetrakis(triphenylphosphine) palladium 0.23g (0.0002mol) to react, raise the temperature to reflux, point the plate to monitor until the reaction is complete, end the reaction, spin the reaction solution to dryness, add 100ml of dichloromethane, make it dissolve completely and Pass through a silica gel column, add 100ml of water to the filtrate, wash with water to separate the organic phase, spin it to dryness, boil it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com