Organic compound and application thereof in preparation of organic electroluminescence element
An organic compound, electroluminescence technology, applied in electrical components, organic chemistry, light-emitting materials, etc., can solve the limitations of OLED technology development, the luminous efficiency and service life of light-emitting components cannot meet the practical requirements, and the blue light materials cannot meet the use requirements. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] The preparation method of compound P01, comprises the steps:
[0117] The first step: preparation of compound Int-1
[0118]
[0119] At room temperature, 0.10mol of acetophenone was dissolved in 300mL of absolute ethanol, and slowly added dropwise to a solution of 0.10mol of 3-bromosalaldehyde or 3-bromo-2-mercaptobenzaldehyde dissolved in absolute ethanol and 0.20mol of The mixed solution of sodium hydroxide dissolved in ethanol, after the addition, was kept and stirred for 4 hours, then concentrated hydrochloric acid was added dropwise to adjust the pH of the reaction solution to 2, concentrated under reduced pressure to dryness, separated and purified by silica gel column to obtain a yellow solid.
[0120] The second step: the preparation of compound Int-2
[0121]
[0122] 90.0mmol of 2-bromoacetophenone and 75.0mmol of the intermediate Int-1 prepared in the first step were dissolved in 150mL of anhydrous THF, and at room temperature, 5.3g (112.5mmol, 85%) o...
Embodiment 2
[0127] The preparation method of compound P05, comprises the steps:
[0128] The first step: preparation of compound Int-3
[0129]
[0130] Referring to the preparation method of the first step in Example 1, replace SM-0 in the first step in Example 1 with salicylaldehyde or o-mercaptobenzaldehyde to prepare intermediate Int-3, a yellow solid.
[0131] The second step: the preparation of compound Int-4
[0132]
[0133] Referring to the preparation method of the second step of Example 1, the intermediate Int-3 prepared in the first step was used to replace the Int-1 in the second step of Example 1 to prepare the intermediate Int-4 as a yellow oil.
[0134] The third step: preparation of compound Int-5
[0135]
[0136] 10.0mmol of the intermediate Int.-4 prepared in the second step was stirred and dissolved with 50ml of acetic acid, 835.0mg (3.9mmol) of potassium iodate and 1.1g (6.6mmol) of potassium iodide were added, the temperature was raised to reflux for 2 ho...
Embodiment 3
[0141] Preparation of compound P31:
[0142]
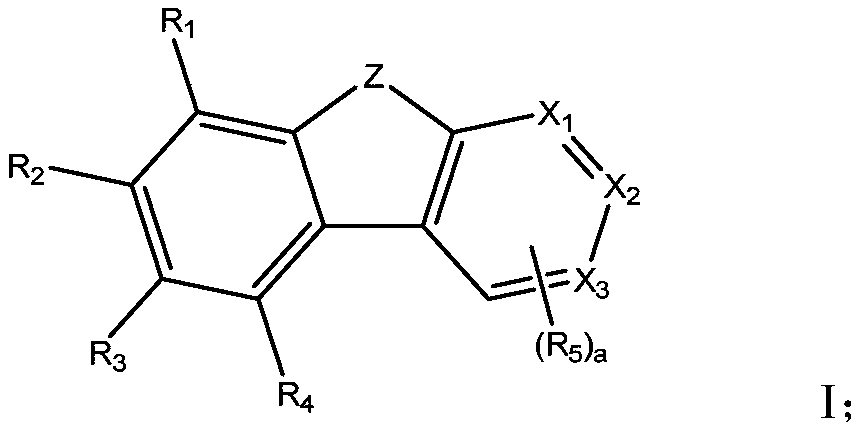
[0143] Under nitrogen protection, 15.6g (44.8mmol) of (3-(2-triphenylene) phenyl) boronic acid, 37.3mmol of Int-5 prepared in the third step of Example 2, 18.0g (0.17mol) of Water and sodium carbonate were mixed, and then 15.0 mg of Pd (PPh 3 ) 4 Catalyst and 100mL of toluene, 50mL of ethanol and 50mL of water, heated to reflux, stirred for 12 hours, cooled to room temperature, diluted with 50mL of water, extracted with toluene, collected organic phase, dried, filtered, and the filtrate was concentrated under reduced pressure. , separated and purified by silica gel column to obtain compound P31, a white solid, the compound where Z is oxygen, HRMS: 624.2346[M+H]; the compound where Z is sulfur, HRMS: 640.2119[M+H]. 1 H-NMR (CDCl 3 , TMS, Z is sulfur compound P31) δ7.16~7.20(m,1H),7.31~7.63(m,14H),7.84~7.98(m,4H),8.18~8.52(m,7H),8.71( s,2H), 9.03(s,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com