Characteristic peptide of peanut allergen protein and application thereof
A technology of characteristic peptides and allergens, applied in the field of food testing, can solve the problems of difficult antibody identification, affect test results, false positives, etc., and achieve the effect of eliminating trypsin inhibitor activity, improving enzymatic hydrolysis efficiency, and achieving good linearity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Screening and determination of embodiment 1 characteristic peptide
[0047] There are 13 known peanut allergenic proteins, and the two with the highest content are Ara h1 and Ara h2, accounting for about 12-16% and 5.9-9.3% of the total peanut protein. Compared with the above two proteins, the content of other allergenic proteins in peanuts is relatively small, and may not provide satisfactory sensitivity in samples containing low concentration levels of peanut proteins, so the present invention can be used in Ara h1 and Ara h2 Appropriate peptides were selected as markers without further investigation of the remaining allergenic proteins.
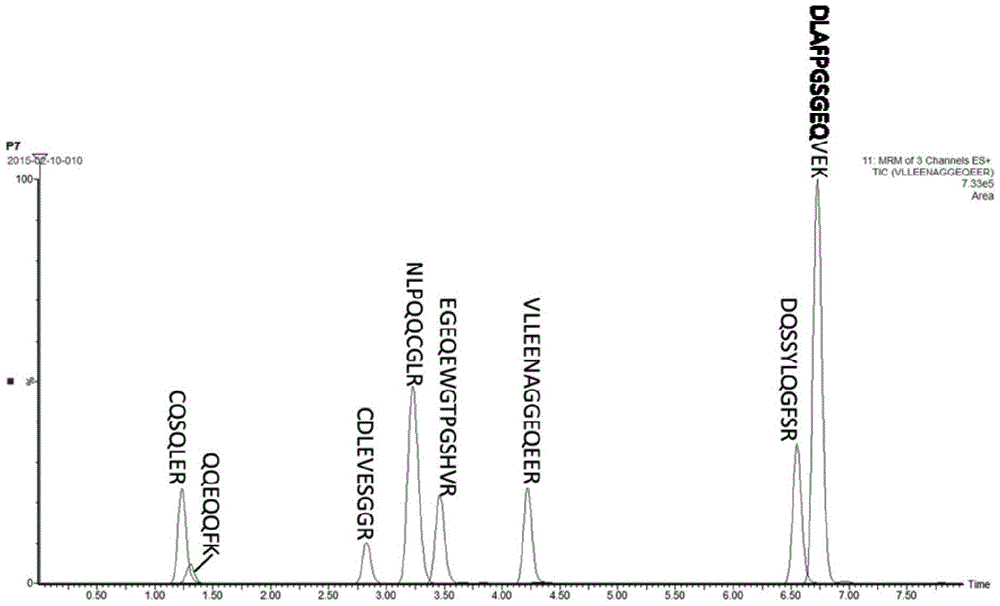
[0048] According to the PeptideMass function provided by Uniprot, the trypsin digestion results show that Ara h1 and Ara h2 can theoretically form 89 and 23 digestion products, respectively. According to the UPLC-Q-ToF detection method provided above, 22 enzymatic polypeptides from Ara h1 and 11 from Ara h2 could be detected, and t...
Embodiment 2
[0068] Example 2 Sample pretreatment (optimization of enzyme digestion conditions)
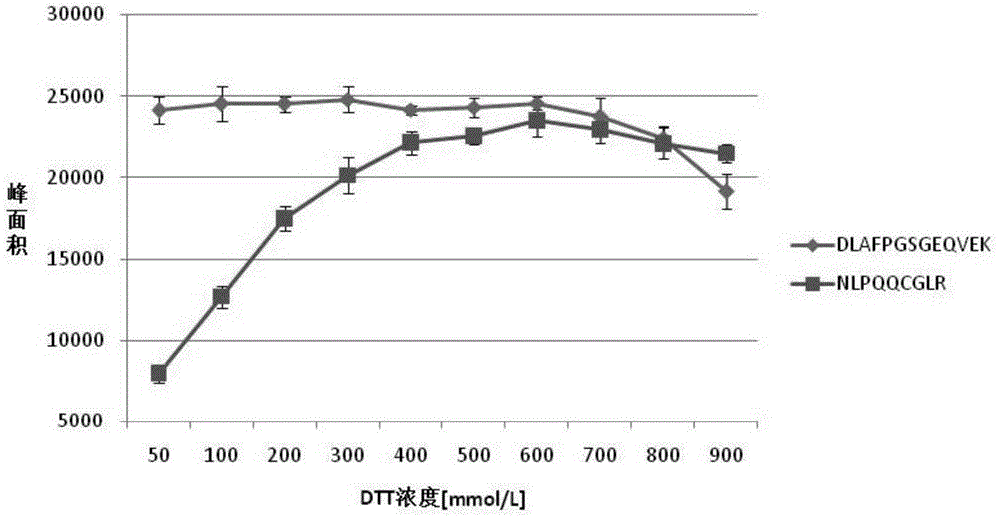
[0069] Studies have shown that Ara h2 has a typical trypsin inhibitor structure. Destroying the spatial structure of the protein by hydrolyzing the disulfide bond can reduce its trypsin inhibitor activity and improve the efficiency of enzymatic hydrolysis. In this embodiment, the degree of protein disulfide bond destruction is increased by increasing the concentration of DTT and optimizing the heating process.
[0070] Prepare DTT with a concentration of 50, 100, 200, 300, 400, 500, 600, 700, 800 and 900mmol / L. In order to fully react the excess DTT and free disulfide bonds, the concentration of the IAA solution is consistent with DTT. And the amount added is three times that of DTT. According to the sample pretreatment method described above, the influence of different DTT concentrations on the digestion efficiency was obtained, and the peak areas of DLAFPGSGEQVEK and NLPQQCGLR were as foll...
Embodiment 3
[0072] Example 3 Detection of Allergen Content in Peanuts of Different Varieties
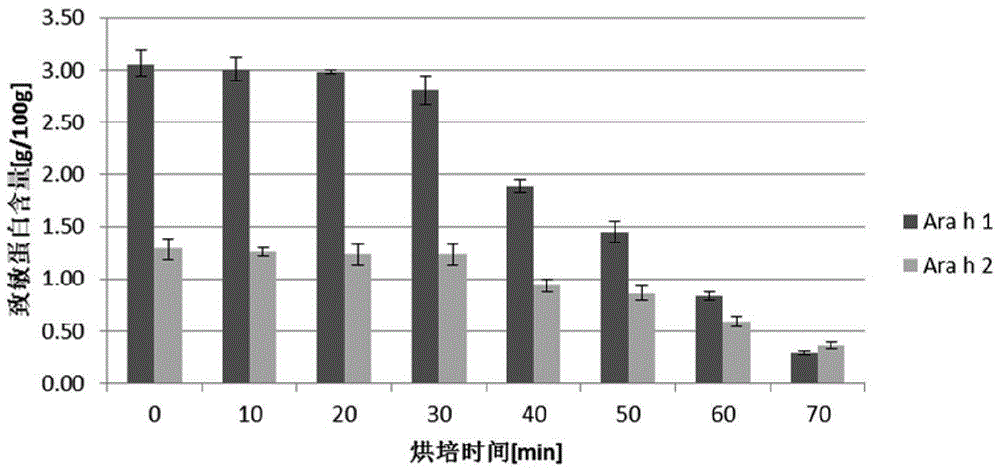
[0073] In the history of peanut planting for more than a thousand years, peanuts have undergone variety improvement, and a large number of peanut varieties have been derived. There are more than 500 varieties of peanuts that can be found only in China. So protein composition may vary between different peanut varieties. In this paper, with the help of the Oil Crops Research Institute of the Chinese Academy of Agricultural Sciences, the 20 most common types of peanuts in China were collected, and the above samples were detected according to the sample pretreatment method, UPLC-TQ-MS analysis method and Kjeldahl method as described above Ara h1, Ara h2 and total protein in peanuts, calculate the percentage of Ara h1 and Ara h2 in total protein in different kinds of peanuts, the results are shown in Table 5.
[0074] Table 5: Detection results of Ara h1 and Ara h2 contents of different varieties of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com