DNA molecule for expressing collagenase in Escherichia coli and method and application of recombinant collagenase
A technology of DNA molecules and Escherichia coli, applied to the DNA molecules of collagenase, the application field of collagenase in degrading collagen, can solve the problems of complex extraction process and high production cost of high-purity collagenase, and achieve significant application prospects and strategies Significant, large yield, significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The construction of embodiment 1 engineering bacterium ColB-RZ1
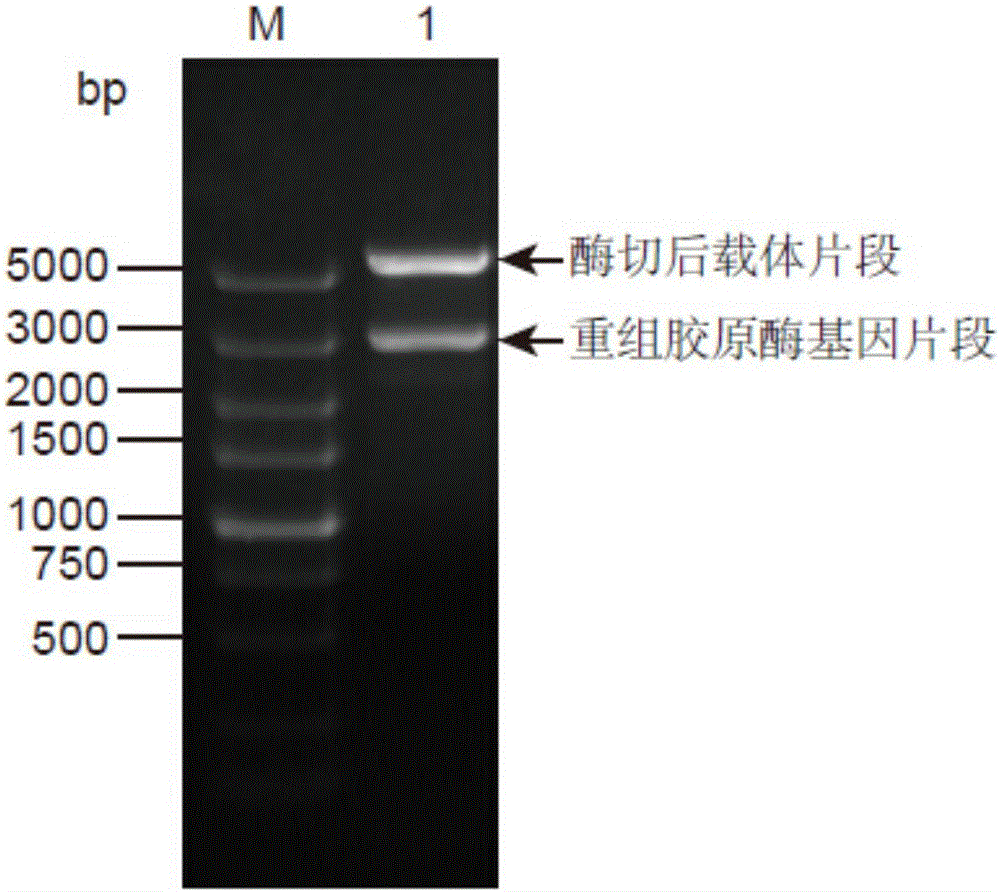
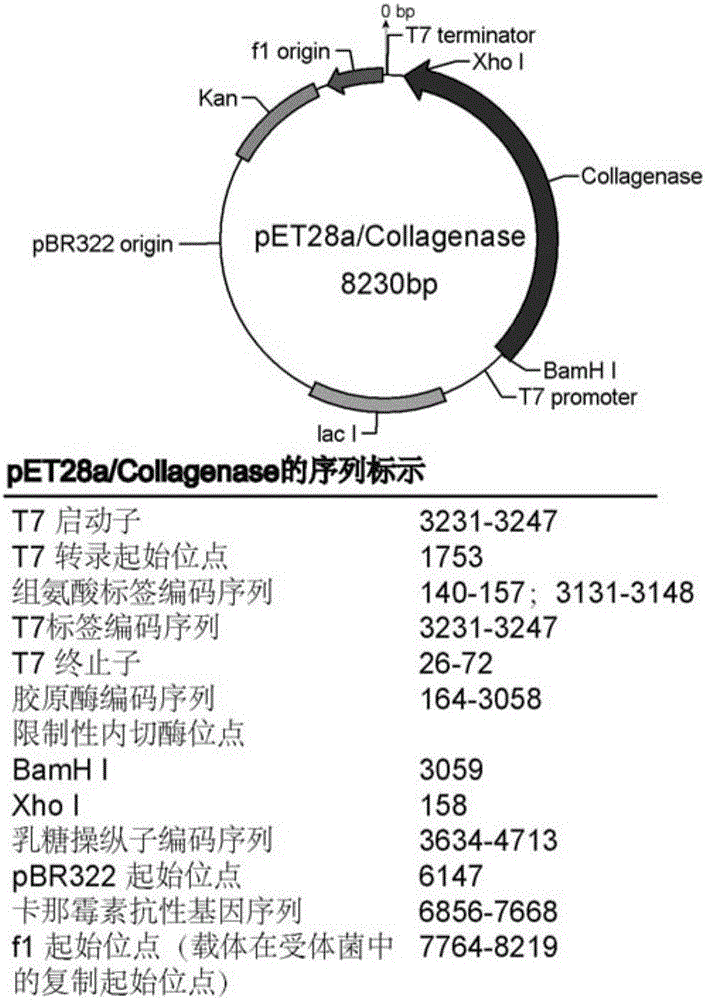
[0041] (1) Construction of pET28a-Collagenase recombinant plasmid
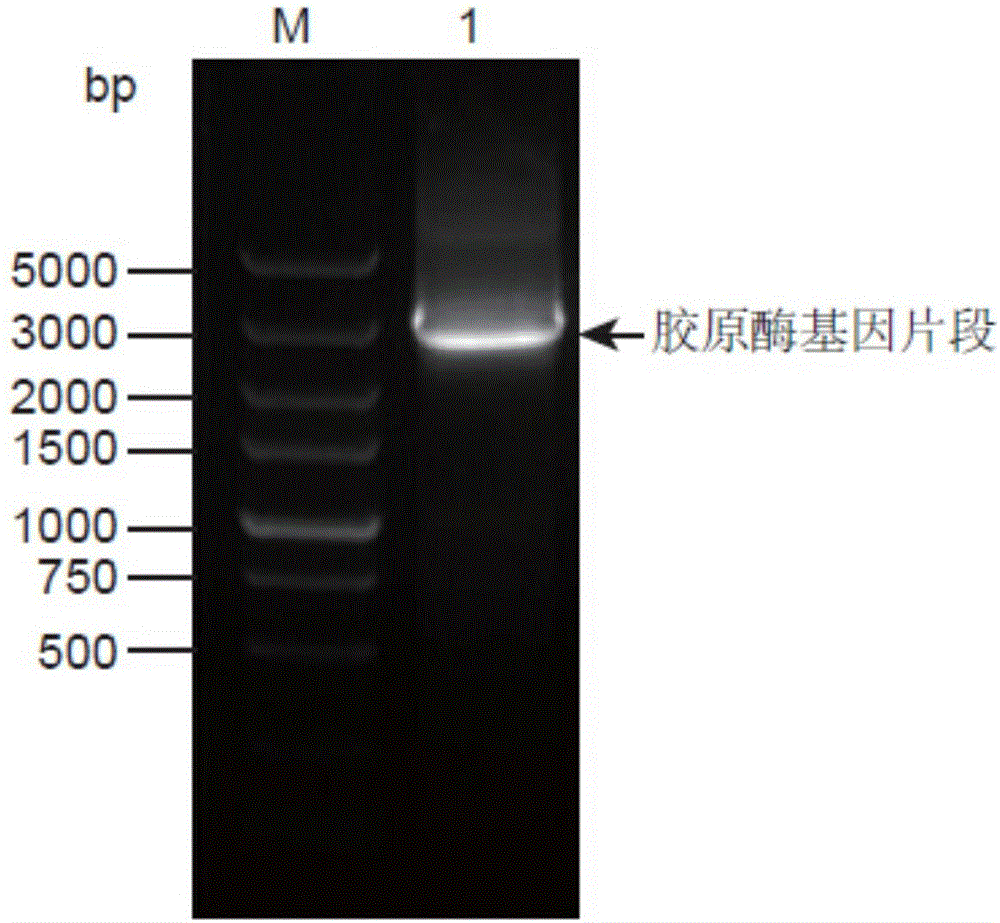
[0042] Using the genomic DNA of Bacillus cereus R75E with the preservation number CGMCC No.8614 in the General Microbiology Center of the China Microbiological Culture Collection Management Committee as a template, the genomic DNA was extracted according to the instructions of the bacterial genomic DNA extraction kit. The upstream primer P1 shown in SEQ ID NO:3 and the downstream primer P2 shown in SEQ ID NO:4 were designed according to the collagenase gene sequence shown in SEQ ID NO:2. The PCR reaction was carried out with the reaction system and conditions shown in Table 1 below.
[0043] Table 1. PCR reaction system for amplifying Bacillus cereus collagenase gene
[0044] Loading order name Volume (μL) 1 10×PCR buffer 5 2 Upstream primer P1 (10mM) 1 3 Downstream primer P2 (10mM) 1 4 MgSO 4 (...
Embodiment 2
[0060] Induction, expression and purification of embodiment 2 recombinant collagenase
[0061] (1) Induced expression and detection of recombinant collagenase
[0062] After culturing the successfully constructed strain ColB-RZ1 in Example 1, a single clone was picked and placed in 5 ml of LB liquid medium containing 34 μg / ml kanamycin, and cultured at 37°C and 150 r / min for 12 hours. The resulting culture was inoculated into 1 L of LB liquid medium containing 34 μg / ml kanamycin, and cultured at 37°C for 2.5-3 hours until the OD600 value of the strain ColB-RZ1 was 0.6-0.8. Add IPTG to the culture solution to The final concentration is 0.1mmol / L. Shake induction culture at 22°C and 150r / min for 16h.
[0063] Centrifuge 1L of the culture medium of ColB-RZ1 strain induced by IPTG at 12000rpm / min at 4°C for 30min to collect the bacterial cells, discard the supernatant, and then resuspend the bacterial pellet with 50ml of buffer 1. Among them, the composition of buffer 1 is: 20m...
Embodiment 3
[0066] Example 3 Activity Analysis of Recombinant Collagenase
[0067] (1) Determination of specific activity of recombinant collagenase
[0068] Determination of recombinant collagen by the general Mandl collagenase activity assay method (Mandl, I., Maclennan, J., D., Howes, E., L., J. Clin. Invest., 12 (1953) 1323-1329.) Enzyme specific activity. In this method, 1 unit of activity (U) is defined as: every milliliter of collagenase reacts with bovine Achilles tendon-derived type I collagen under the reaction conditions of 37°C and pH value of 7.4, and each unit of activity produced within 5 hours 1 μmoL free amino acid corresponds to 1 activity unit (1U). The specific activity of collagenase is represented by the ratio (U / mg) of the total number of enzyme activity units (U) per milliliter of collagenase to the amount of collagenase protein per milliliter (mg). The specific steps of viability determination are:
[0069] a, L-glycine standard curve drawing
[0070] ①Use bu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific vitality | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com