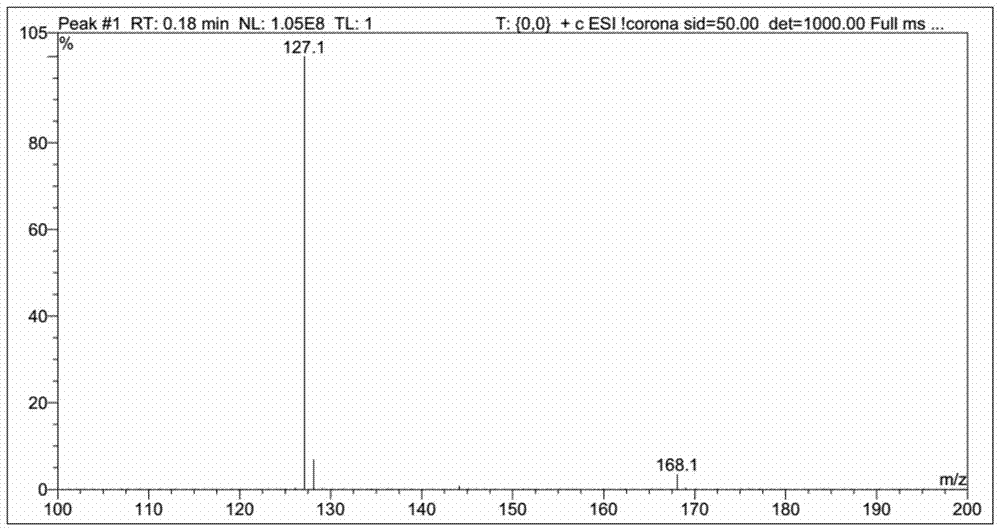
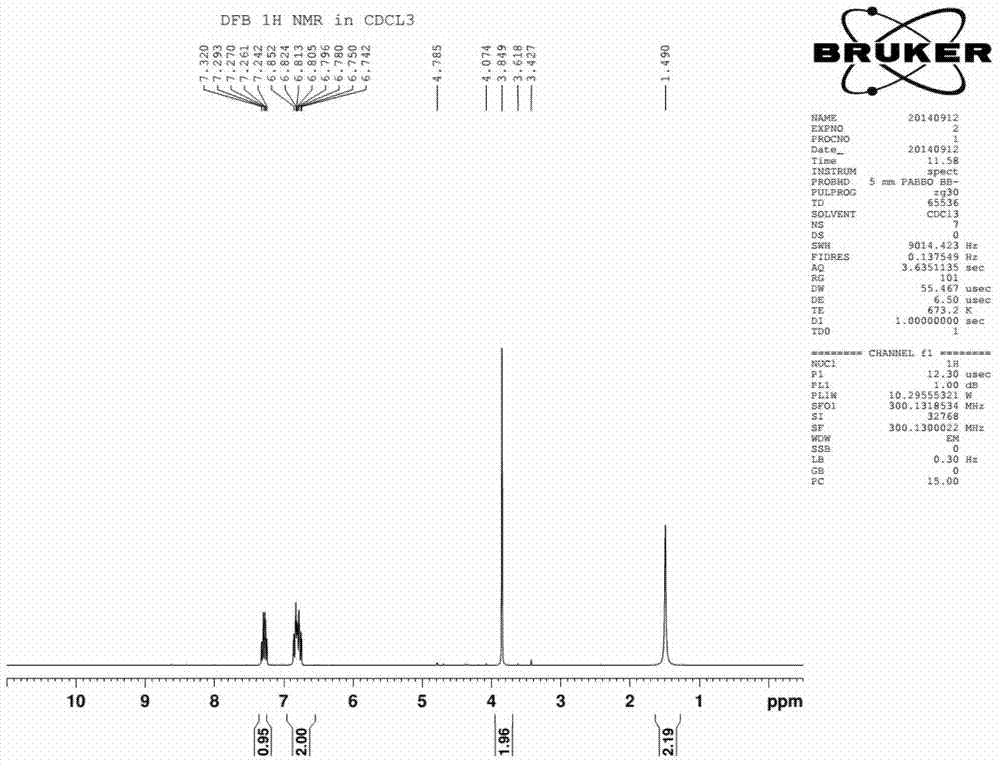
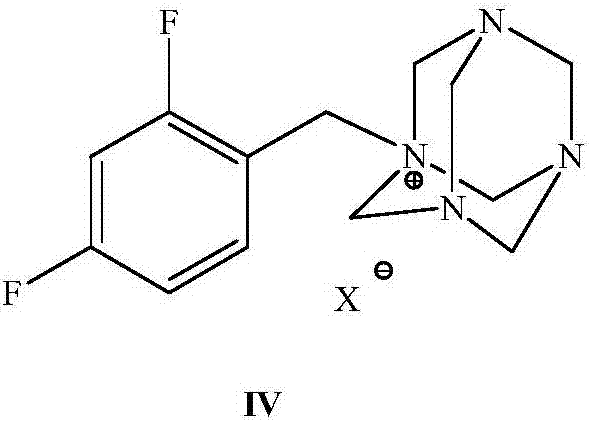
The synthetic method of 2,4-difluorobenzylamine
The technology of a kind of difluorobenzylamine and its synthetic method is applied in the field of synthetic medicine and agricultural chemicals, which can solve the problems of unsafe reagents, expensive raw materials, harsh operating conditions, etc., and achieve cheap raw materials and reagents and simple and convenient operation methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The synthetic method of 2,4-difluorobenzylamine comprises the following three steps:
[0038] Synthesis of compound (Ⅲ)
[0039]
[0040] Add 250ml of acetonitrile, 35g (1.17mol) octa-formaldehyde and 45g (0.39mol) m-difluorobenzene into a 500ml four-necked flask, slowly pour 200ml (2.4mol) of 33% concentrated hydrochloric acid under stirring, then add 21g (0.16 mol) of zinc chloride, and the mixture was slowly heated to reflux, and the solid gradually dissolved and reacted for 8 hours. After the HPLC detection was complete, the temperature was lowered to room temperature, and acetonitrile was removed under reduced pressure at 45°C to obtain a two-phase mixture. Chloromethane was extracted twice, and the organic layers were combined, dried with anhydrous sodium sulfate, and then filtered with suction, and the filtrate was concentrated under reduced pressure to obtain a transparent liquid, which was then distilled under reduced pressure with a refrigerant at -5°C-0°C,...
Embodiment 2
[0051] The synthetic method of 2,4-difluorobenzylamine comprises the following three steps:
[0052] Synthesis of compound (Ⅲ)
[0053]
[0054] Add 250mL THF, 35g (1.17mol) pentaformaldehyde and 45g (0.39mol) m-difluorobenzene into a 500ml four-neck flask, slowly pour 200mL (2.4mol) of 33% concentrated hydrochloric acid under stirring, then add 25g (0.19mol) mol) of zinc chloride, and the mixture was slowly heated to reflux, and the solid gradually dissolved and reacted for 6 hours. After the HPLC detection was complete, the temperature was lowered to room temperature, and THF was removed under reduced pressure at 45°C to obtain a two-phase mixture. Extracted twice with methyl chloride and combined the organic layers, dried with anhydrous sodium sulfate, then filtered with suction, and concentrated the filtrate under reduced pressure to obtain a transparent liquid, then distilled under reduced pressure with a refrigerant at -5°C-0°C, collected 60°C / 10mmHg The distillate o...
Embodiment 3
[0062] The synthetic method of 2,4-difluorobenzylamine comprises the following three steps:
[0063] Synthesis of compound (Ⅲ)
[0064]
[0065] Add 250mL of dioxane, 45.5g (1.52mol) of paraformaldehyde and 45g (0.39mol) of m-difluorobenzene in a 500ml four-necked flask, and slowly pour 300mL of 47% hydrobromic acid (2.63mol) under stirring, Then add 32g (0.20mol) of ferric chloride, and slowly heat the mixture to reflux, the solid gradually dissolves and reacts for 7 hours, after HPLC detects that the reaction is complete, cool to room temperature, and remove dioxane under reduced pressure at 45°C to obtain The two-phase mixture was extracted twice by adding 150ml of dichloromethane, and the organic layers were combined, dried with anhydrous sodium sulfate, and then filtered with suction, and the filtrate was concentrated under reduced pressure to obtain a transparent liquid, which was then decompressed with a refrigerant at -5°C-0°C. Pressure distillation, collecting fra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com