Method for preparing 2-amino-1-naphthalene sulfonic acid
A technology of naphthalenesulfonic acid and amino, which is applied in the field of preparation of 2-amino-1-naphthalenesulfonic acid, can solve problems such as ammoniated side reactions, and achieve the effects of high reaction efficiency, high purity, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
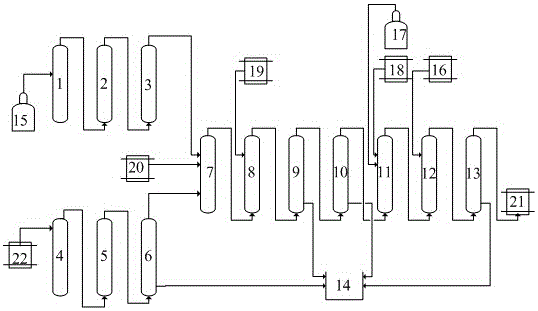
[0012] Such as figure 1 Shown a kind of method for preparing 2-amino-1-naphthalenesulfonic acid, the method comprises raw material reaction tower 1, the first separation reaction tower 2, condensation reaction tower 3, subtemperature reaction tower 4, precipitation reaction tower 5, fractionation Reaction tower 6, first synthesis reaction tower 7, neutralization reaction tower 8, second separation reaction tower 9, distillation reaction tower 10, second synthesis reaction tower 11, acidification reaction tower 12, dehydration reaction tower 13, wastewater treatment method 14 , hydrogen chloride gas storage tank 15, hydrogen chloride liquid storage tank 16, ammonia gas storage tank 17, ammonium sulfate liquid storage tank 18, sodium sulfate liquid storage tank 19, 2-naphthol storage tank 20, finished product storage tank 21, xylene storage Tank 22; wherein, the gas outlet of the hydrogen chloride gas storage bottle 15 is connected to the gas inlet 1 of the raw material reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com