Bodipy compound and application thereof
A technology of fluoroboropyrrole and compound, which is applied in the field of fluorescent probes for detecting nitrosyl hydrogen, can solve the problems of probe interference, interference detection, lack of sensitive and specific detection, etc., to reduce interference and improve detection accuracy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1. Preparation of fluoroborate pyrrole compound:
specific Embodiment
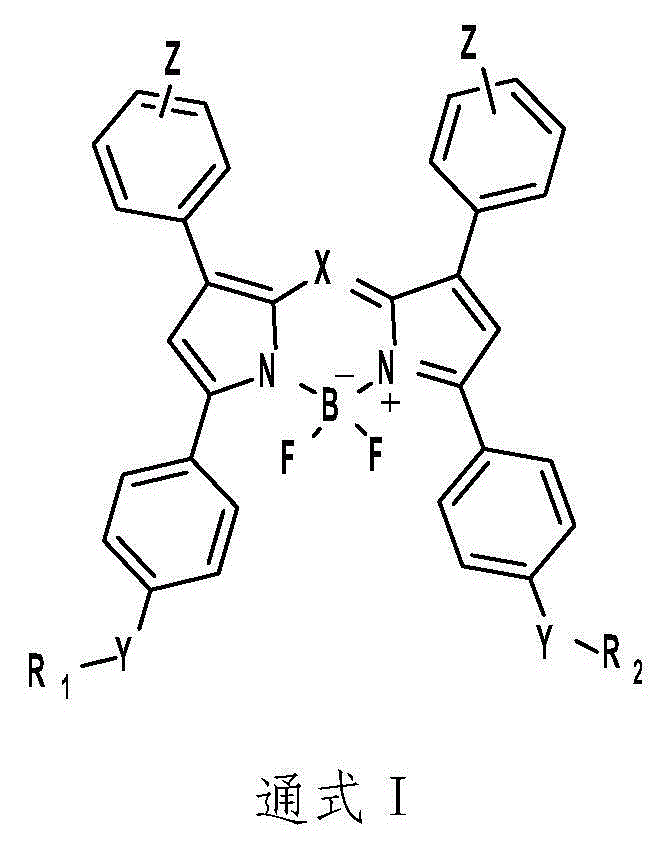
[0045] Fluoropyrrole compounds shown in general formula I react with commercially available p-hydroxyacetophenone and benzaldehyde to synthesize the corresponding fluorophore BODIPY, and then modify different positioning groups on the corresponding positions of the fluorophore. Finally, the fluorophore modifying the positioning group is reacted with 2-(diphenylphosphine)benzoic acid in dichloromethane solvent to generate the corresponding fluoroboryrrole compound by DMAP and EDC catalysis. Specific examples are as follows:
[0046] Preparation of a compound of formula:
[0047]Under argon protection, BODIPY fluorophore (52.9mg, 0.1mmol) and 2-(diphenylphosphine) benzoic acid (61.2mg, 0.2mmol), DMAP (24.4mg, 0.2mmol), EDCI (19.2mg, 0.1 mmol) was dissolved in a 50ml flask filled with 20ml of dry dichloromethane, stirred at room temperature for 24h, followed by TLC. The crude product was neutralized by hydrobromic acid, washed with a saturated NaBr solution until neutral, extra...
Embodiment 2
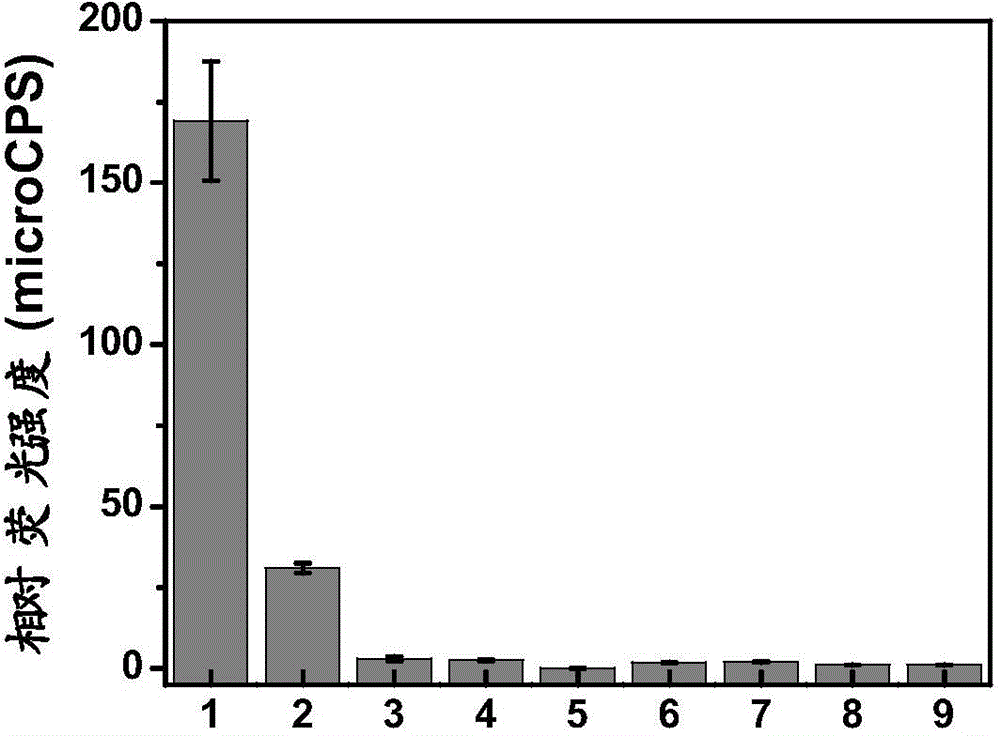
[0061] The prepared compound of formula two is used as a probe in water system, simulated physiological environment and intracellular to detect HNO, simulated physiological conditions, the following experiments are all carried out under the condition of pH=7.4 (HEPES buffer solution, concentration is 40mM ), and the probe concentration was 10 μM.
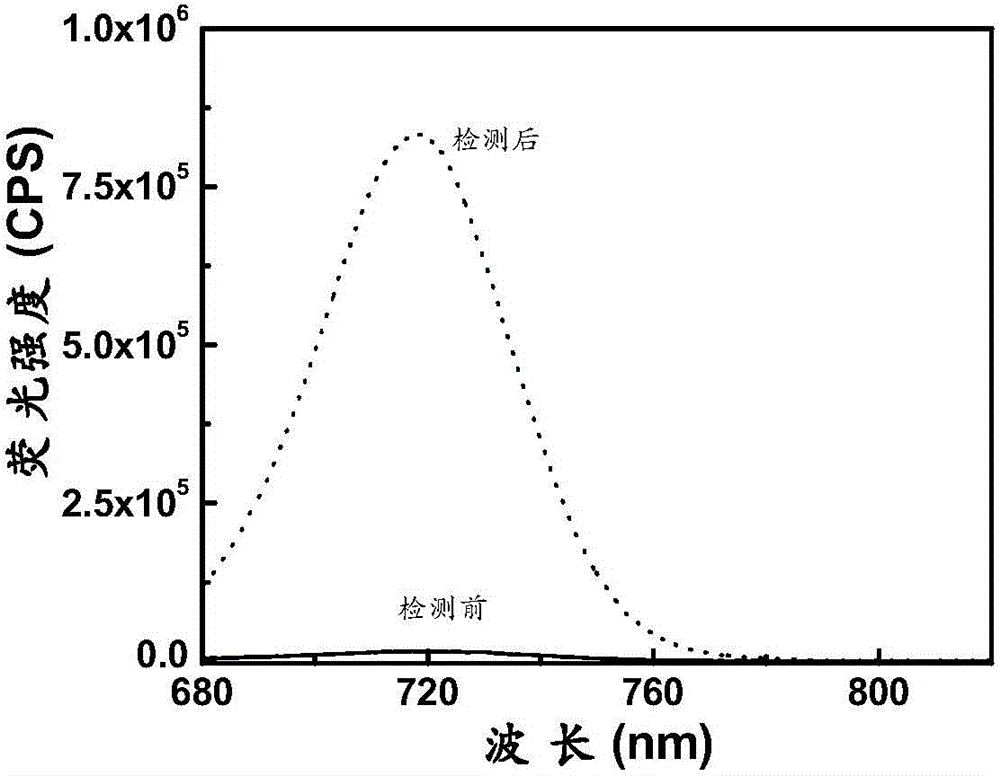
[0062] The spectral response of the compound of Formula 2 prepared above to HNO; add 10 μM of the compound of Formula 2 to a 10 ml colorimetric tube, then add 40 mM HEPES, then add 10 μM of HNO, dilute the volume to 10 ml with ultrapure water, shake the solution to balance for 10 minutes, and then The above working solution was added into a fluorescent dish to measure the fluorescence spectrum. The fluorescence spectrum changes as figure 1 shown. Depend on figure 1 The compound shown can be used to realize the detection of HNO in vivo. At the same time, the structure of the product after the reaction of the probe provided in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com