Anti-human NMP-22 monoclonal antibody and detection kit thereof
A technology of NMP-22 and monoclonal antibody, applied in the field of molecular immunology, can solve the problems of low sensitivity, time-consuming and labor-intensive kit operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
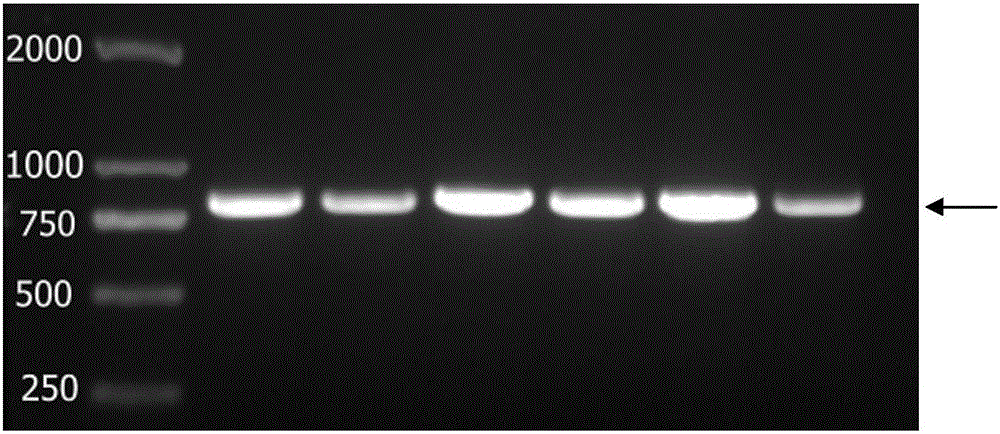
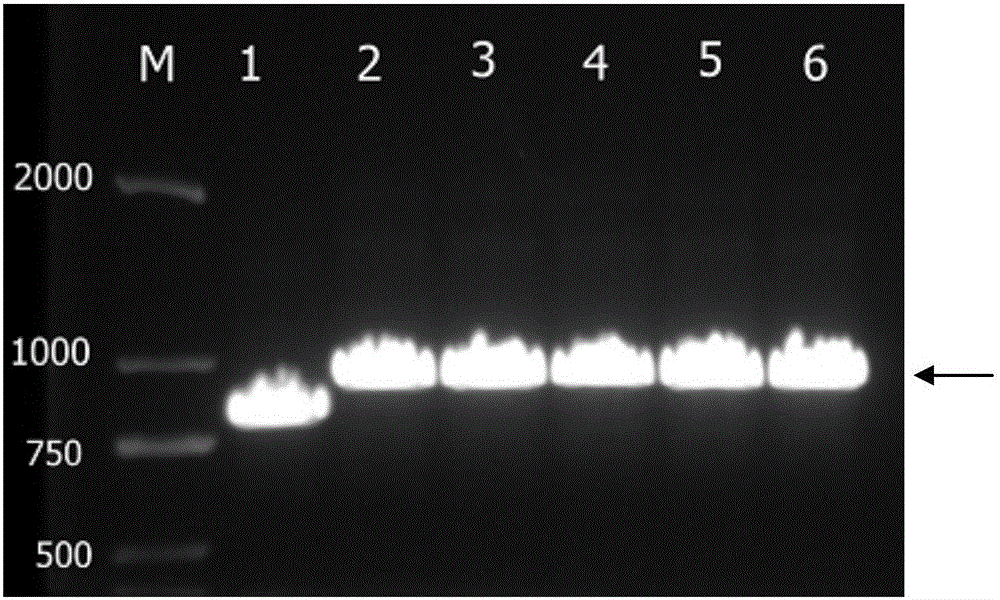
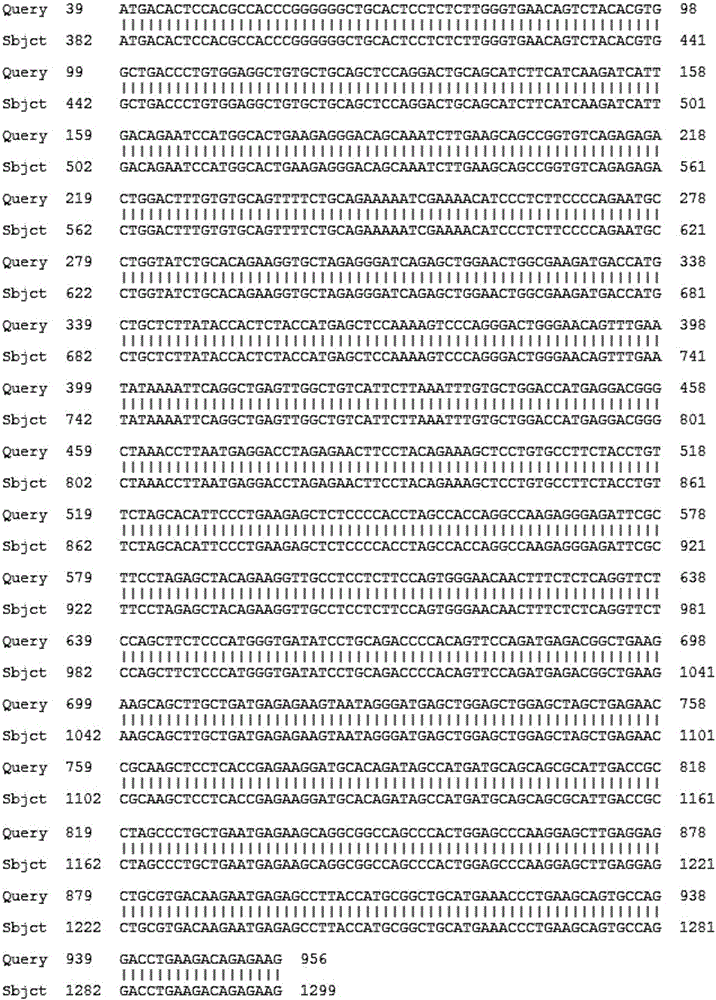
[0050] Example 1. Preparation and purification of recombinant human NMP-22 protein
[0051] 1. According to the instruction manual of EASYspin Whole Blood RNA Rapid Extraction Kit (Cat. No. RN06) produced by Beijing Bomed Gene Technology Co., Ltd., extract whole blood RNA.
[0052] 2. According to the instruction manual of the Transcript First-strand cDNA Synthesis SuperMix kit (product number AT301-02) produced by Beijing Quanshijin Biotechnology Co., Ltd., the following reaction system is used to complete the operation:
[0053] cDNA first strand synthesis reaction system
[0054]
[0055] After incubating at 42°C for 30 minutes, reverse transcriptase was inactivated at 85°C for 5 minutes to terminate the reaction.
[0056] Among them, Oligo(DT)18, 2× reaction mixture solution, and enzyme mixture are all from the above kit, and RNA is from the whole blood RNA extracted in step 1.
[0057] 3. Perform PCR reaction to amplify NMP-22 target gene according to the following r...
Embodiment 2
[0071] Example 2. Preparation of anti-human NMP-22 mouse monoclonal antibody
[0072] 1. Select 5 female Balb / c mice aged 6 weeks and weighing about 20 g. The recombinant protein prepared in Example 1 was used for immunization. For the first immunization, take 100 μg of protein and add 0.01M PBS to 500 μl, emulsify with an equal volume of Freund’s complete adjuvant, and inject subcutaneously at multiple points after the emulsification is sufficient. On the 14th and 35th days, the second and third immunization doses were the same as the first immunization, emulsified with Freund's incomplete adjuvant, and then subcutaneously injected at multiple points after emulsification was sufficient. One week after the three immunizations, blood from the tail vein of the mice was collected, and the recombinant antigen was coated with the serum titer by the indirect Elisa method, and the mouse with the highest titer was selected (B mice, see Figure 9 ) spleen for cell fusion. For booste...
Embodiment 3
[0080] Example 3: Preparation of Human NMP-22 Chemiluminescent Kit
[0081] 1. Prepare a microwell plate coated with NMP-22 primary antibody:
[0082] a) Coating: 0.05M carbonate buffer solution with a pH value of 9.6 and the mouse monoclonal antibody with the clone number 5E8 prepared in Example 2 at an appropriate concentration were used to prepare coating solutions with different concentrations. Select the coating concentration of 5 μg / ml, 2.5 μg / ml, 1.25 μg / ml, 0.625 μg / ml, and load the coating solution on the microplate at 120 μl / well, and coat at 4°C for 12 hours;
[0083] Carbonate buffer standard formula:
[0084]
[0085] b) Washing plate: dilute 20 times concentrated lotion to 1 times concentration, wash the plate twice with 1 times concentration lotion; standard formula of 20 times concentrated lotion:
[0086]
[0087] c) Blocking: Load the blocking solution (disodium hydrogen phosphate 5.8g, sodium dihydrogen phosphate 0.593g, sodium chloride 8.0g, goat se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com