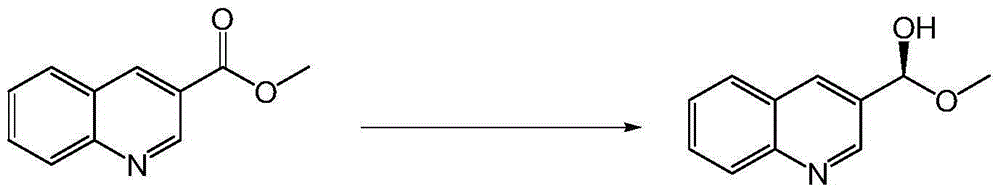Carbonyl reductase and application of same in synthesis of chiral hydroxyl compound
A technology of carbonyl reductase and reductase, which is applied in the field of bioengineering, can solve the problems of harsh reaction conditions and low yield, and achieve the effects of mild reaction conditions, high product concentration and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
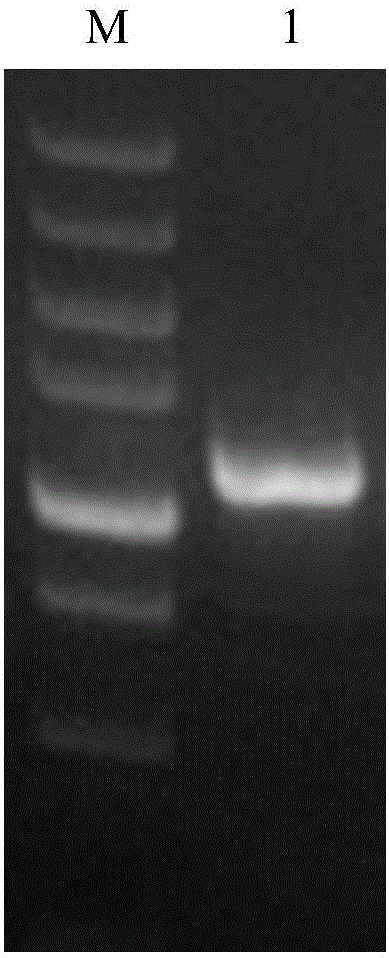
[0042] Cloning of Example 1 Carbonyl Reductase Gene
[0043] The sequence was synthesized according to the known sequence of 3-oxoacyl-ACP reductase of S. albus (see SEQ ID No: 1 for the amino acid sequence), and the corresponding DNA sequence was obtained as shown in SEQ ID No: 2. The gene was cloned into the NdeI / HindIII site of pET28a to construct the corresponding expression plasmid pABI-ADH-SA, which was verified by PCR and electrophoresis. The primers used were shown in SEQ ID No:3 and 4. Transform into Escherichia coli BL21 (DE3) competent cells to obtain the corresponding recombinant strain sABI-ADH-SA.
Embodiment 2
[0044] Embodiment 2 Expression of recombinant carbonyl reductase
[0045] Inoculate the recombinant strain sABI-ADH-SA into LB shake flasks containing kanamycin and cultivate overnight at 37°C, and inoculate the overnight culture into 1L LB shake flasks at a ratio of 5%, and cultivate to OD at 37°C 600 0.6-0.8, add IPTG to a final concentration of 1 mM, lower the temperature to 30°C and continue culturing for 16 hours. The bacterial cells were collected by centrifugation at 4000rpm, resuspended with 100mL PBS (pH 7.5) buffer solution, ultrasonically disrupted, and centrifuged at 12000rpm for 30min to obtain the supernatant which was the crude enzyme solution.
[0046] The method of measuring enzyme activity (U) is: in 2mL reaction solution, add 2mM acetophenone as substrate, 0.1mM NADH as cofactor, then add 20μL crude enzyme solution, measure OD within 1 minute 340 The decrease is ΔA 340 . Specific enzyme activity per mL of enzyme solution U=ΔA 340 ×1000 / (6220×20), that i...
Embodiment 3
[0050] The asymmetric reduction reaction of embodiment 3 prochiral carbonyl substrates
[0051]
[0052] Suspend 40 g of methyl quinoline-3-carboxylate in 500 mL of sodium phosphate buffer and wash with saturated Na 2 CO 3 The pH of the solution was adjusted to 6.0, and 200 mL of the crude enzyme solution prepared in Example 2, 100 g of glucose and 1000 U of glucose dehydrogenase (purchased from sigma), and 0.2 mmol / L of NAD were added + , add sodium phosphate buffer to 1L, stir and react at 30°C for 24 hours, saturate with Na 2 CO 3 The pH of the reaction was controlled at about 6.0, and the progress of the reaction was detected by TLC. After the reaction, adjust the pH to 2.0, raise the temperature to 70°C and heat for 1 hour to denature the protein, add diatomaceous earth to filter to remove the denatured protein, and then adjust the pH to 13.0; extract twice with an equal volume of n-butanol, and wash with an equal volume of n-butanol The residue was filtered once, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com