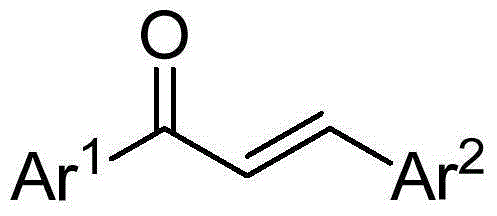
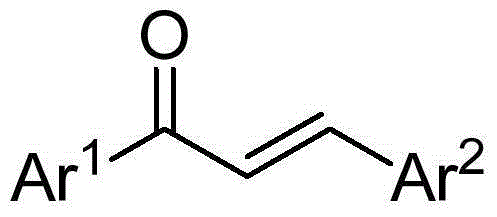
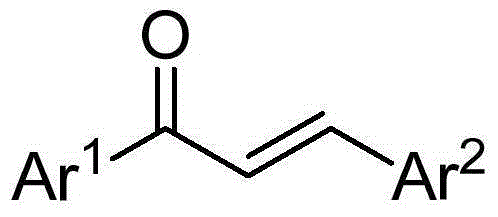
Synthetic method for 1,3,5-triarylbenzene compound
A synthesis method and technology of triarylbenzene, which are applied in the field of synthesis of 1,3,5-triarylbenzene compounds, can solve the problems of difficult preparation of substrates, harsh reaction conditions, narrow substrate range, etc. Easy post-processing and high applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of 1,3,5-triphenylbenzene
[0023] Add 0.25mmol of chalcone, 1.5mmol of sodium tert-butoxide, and 2.0mL of dimethyl sulfoxide into a 10mL reaction tube, place in an oil bath at 60°C, and expose to air for 5h. Stop the reaction and cool to room temperature. The reaction solution was diluted with ethyl acetate, extracted three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, and separate by column chromatography to obtain 28.2 mg of the target product, with a yield of 77%. The NMR characterization of this compound is as follows: 1 HNMR (400MHz, CDCl 3 )δ7.78(s,3H),7.73–7.67(m,6H),7.51–7.44(m,6H),7.42–7.35(m,3H); 13 CNMR (100MHz, CDCl 3 )δ142.35, 141.16, 128.84, 127.54, 127.36, 125.17.
Embodiment 2
[0025] Preparation of 1-(4-methoxyphenyl)-3,5-diphenylbenzene
[0026] Add 0.25mmol of 4'-methoxychalcone, 1.5mmol of sodium tert-butoxide, and 2.0mL of dimethyl sulfoxide into a 10mL reaction tube, place it in an oil bath at 60°C, and expose it to the air for 5h . Stop the reaction and cool to room temperature. The reaction solution was diluted with ethyl acetate, extracted three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Dried, filtered, and separated by column chromatography to obtain 25.6 mg of the target product with a yield of 63%. The NMR characterization of this compound is as follows: 1 HNMR (400MHz, CDCl 3 )δ7.78(s,1H),7.76–7.71(m,1H),7.68(d,J=8.7Hz,1H),7.51(t,J=7.6Hz,1H),7.42(t,J=7.3 Hz,1H),7.05(d,J=8.7Hz,1H),3.91(s,1H); 13 CNMR (100MHz, CDCl 3 )δ159.40, 142.34, 141.94, 141.28, 133.68, 128.84, 128.39, 127.51, 127.38, 124.79, 124.66, 114.32, 55.40.
Embodiment 3
[0028] Preparation of 1-(4-tert-butylphenyl)-3,5-diphenylbenzene
[0029] Add 0.25mmol of 4'-tert-butylchalcone, 1.5mmol of sodium tert-butoxide, and 2.0mL of dimethyl sulfoxide into a 10mL reaction tube, place it in an oil bath at 60°C, and expose it to the air for 5h . Stop the reaction and cool to room temperature. The reaction solution was diluted with ethyl acetate, extracted three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Dried, filtered, and separated by column chromatography to obtain 27.4 mg of the target product with a yield of 49%. The NMR characterization of this compound is as follows: 1 HNMR (400MHz, CDCl 3 )δ7.81(dd, J=6.4,1.3Hz,3H),7.78–7.71(m,4H),7.68(d,J=8.3Hz,2H),7.53(dd,J=15.5,8.1Hz,6H ),7.42(t,J=7.3Hz,2H),1.42(s,9H); 13 CNMR (100MHz, CDCl 3 )δ150.63, 142.28, 142.23, 141.26, 138.27, 128.86, 127.52, 127.37, 127.03, 125.83, 125.10, 124.96, 34.61, 31.41.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com