Process for refining caprolactam
A technology of caprolactam and crude caprolactam, which is applied in the field of caprolactam refining technology, can solve the problems of insufficient utilization of ion exchange resin exchange capacity, increased energy consumption in the refining process, and shortened resin regeneration cycle, so as to save costs and resources, and reduce regeneration Frequency, the effect of avoiding waste water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
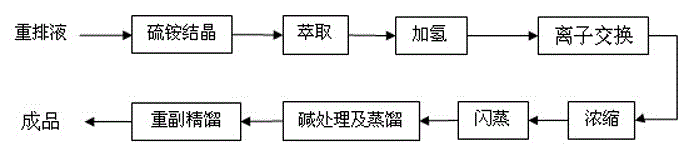
[0040] The extracted caprolactam aqueous solution is subjected to a hydrogenation operation under the action of a Raney nickel catalyst to remove unsaturated by-products in the caprolactam. The temperature of hydrogenation reaction is 75°C, H 2 The pressure is 700kPa, the reaction time is 30min, and the catalyst concentration is about 0.05mg Raney nickel per liter.
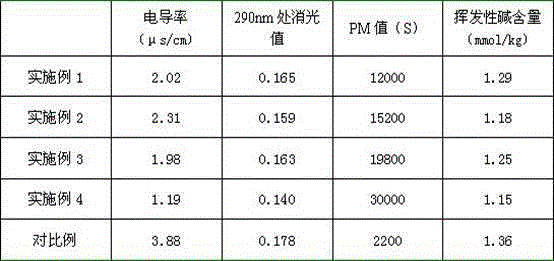
[0041] The caprolactam aqueous solution after hydrogenation is cooled to 45°C, pumped countercurrently into the ion exchange adsorption device, wherein the ion exchange device takes the pretreated wet cationic resin and wet anion resin according to the ratio of 1:2, and adopts anion- Yang-yin (volume ratio 1:1:1) is loaded in series. The temperature of ion exchange adsorption is controlled to be 45°C, and the residence time is 45min. Measure the conductivity, absorbance value at 290nm, volatile alkali content and potassium permanganate absorbance value of the discharge liquid. When the extinction value at 290nm...
Embodiment 2
[0043] Repeat the method described in Example 1, the difference is: the temperature of hydrogenation reaction is 90 DEG C, H 2 The pressure is 700kPa, the reaction time is 60min, and the catalyst concentration is about 0.15mg Raney nickel per liter. The results of various quality indexes of caprolactam after hydrogenation and ion exchange are shown in Table 2, and the operating cycle and dynamic adsorption capacity are shown in Table 3.
Embodiment 3
[0045] Repeat the method described in Example 1, the difference is: the temperature of hydrogenation reaction is 105 DEG C, H 2 The pressure is 700kPa, the reaction time is 90min, and the catalyst concentration is about 0.10mg Raney nickel per liter. The results of various quality indexes of caprolactam after hydrogenation and ion exchange are shown in Table 2, and the operating cycle and dynamic adsorption capacity are shown in Table 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Dynamic adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com