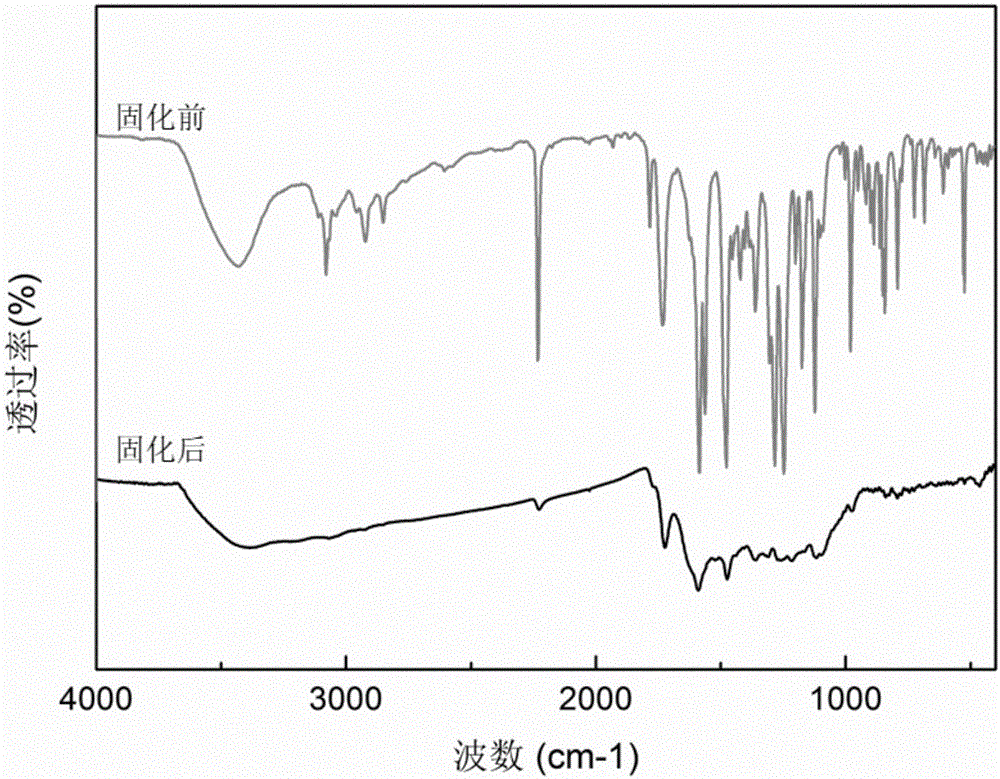
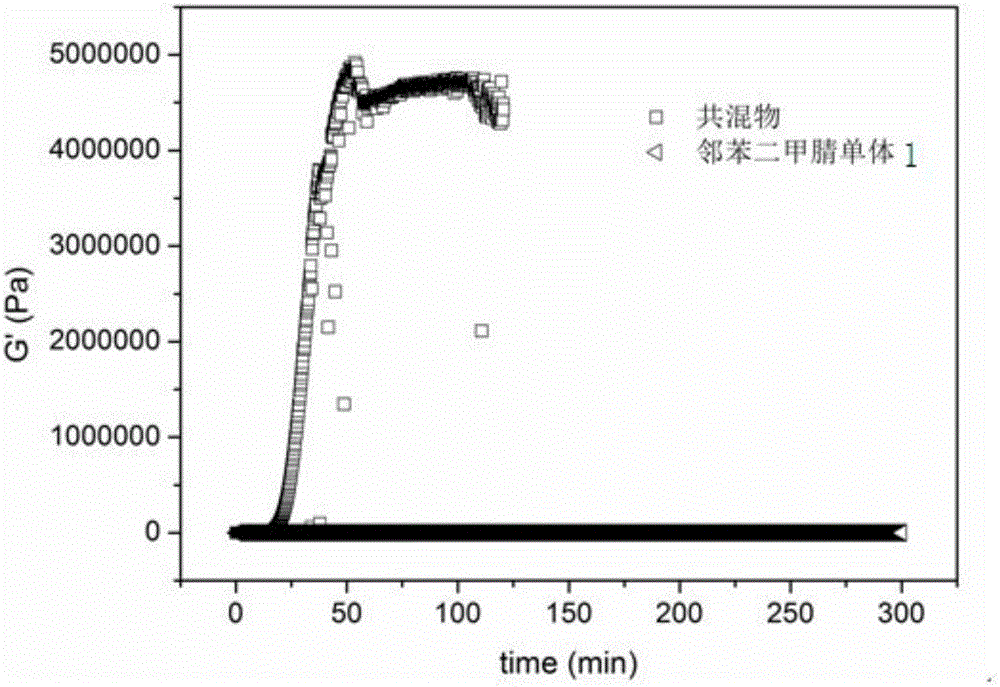
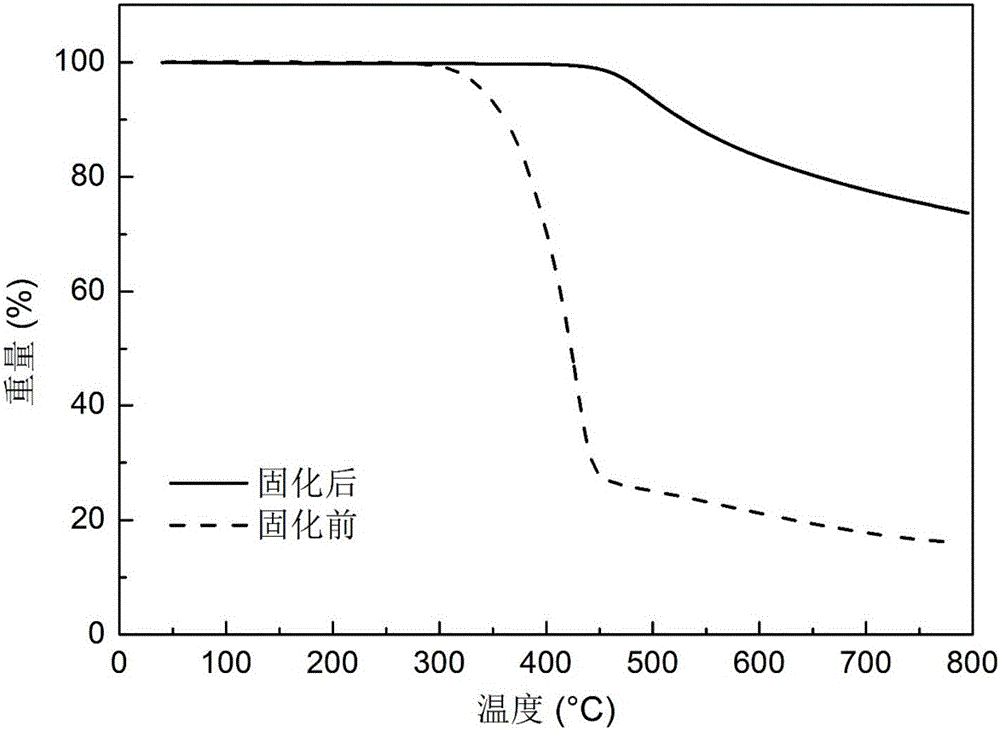
Application of active-hydrogen-containing heterocyclic compound to preparation of phthalonitrile resin
A technology of phthalonitrile resin and heterocyclic compound, which is applied in the application field of active hydrogen-containing heterocyclic compound in the preparation of phthalonitrile resin, and can solve the problem of reducing resin performance, thermal stability and thermal oxygen stability Poor performance and other problems, to achieve thermal stability and thermo-oxygen stability improvement, to avoid the reduction of mechanical properties and thermal properties, and excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This embodiment is an active hydrogen purine structure accelerated phthalonitrile resin:
[0038] (1) Synthesis of active hydrogen-containing heterocyclic compound A1
[0039] Add adenine (Cas: 73-24-5, 0.02mol, 2.70g) and 60ml of refined NMP into a 100mL three-necked flask, stir for 10 minutes, then add pyromellitic anhydride (PMDA, 0.01mol, 2.18g) After stirring at room temperature for 7 h, 20 mL of purified toluene was added to the reaction system, and the temperature of the system was raised to 145° C. under reflux, and the reaction was carried out at this temperature for 7 h. When no more water is produced in the system, raise the temperature to 180°C and separate the toluene in the system. When the temperature of the reaction system rises sharply, it means that the toluene has been basically removed. Turn off the heating and stirring. When the system drops to room temperature, use Precipitate with hot water, stir wash, filter the filter cake, wash the filter cake...
Embodiment 2
[0051] This embodiment is that imidazole accelerates the curing of phthalonitrile resin:
[0052] (1) Synthesis of active hydrogen-containing heterocyclic compound A2
[0053] Add (Cas: 4919-03-30.02mol, 1.66g) and 60ml refined NMP into a 100mL three-necked flask, stir for 10 minutes, then add 3,3,4,4-biphenyldianhydride (BPDA, 0.01mol, 2.94 g), after stirring at room temperature for 7 h, 20 mL of purified toluene was added to the reaction system, and the temperature of the system was raised to 145° C. to reflux, and reacted at this temperature for 5 h. When no more water is produced in the system, raise the temperature to 180°C and separate the toluene in the system. When the temperature of the reaction system rises sharply, it means that the toluene has been basically removed. Turn off the heating and stirring. When the system drops to room temperature, use Precipitate with hot water, stir wash, and filter the filter cake, then wash the filter cake three times with water, s...
Embodiment 3
[0065] This embodiment is that pyrimidine can accelerate the solidification of phthalonitrile resin:
[0066] (1) Synthesis of active hydrogen-containing heterocyclic compound A3
[0067] Add ODPA (0.10mol, 31.02g) and 124ml NMP into a 500ml three-necked flask, heat the oil bath until ODPA can be completely dissolved (the temperature is about 70°C, the system is light yellow and transparent), and then lower the temperature to room temperature. Cytosine (Cas: 71-30-7, 0.20 mol, 22.22 g) was added to the reaction solution at room temperature, and stirred at room temperature for 6 h. Pyrimidine acetic anhydride (0.60mol, 62.19g, 98.5%wt) and pyridine (0.60mol, 47.70g, 99.5%wt) were added, and the temperature of the system was raised to 120°C, and reacted at this temperature for 3h. But the system is lowered to room temperature, precipitated with hot water, stirred and washed, and the filtered filter cake is washed three times with water and twice with methanol. After suction fil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| carbon residual rate | aaaaa | aaaaa |
| carbon residual rate | aaaaa | aaaaa |
| carbon residual rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com