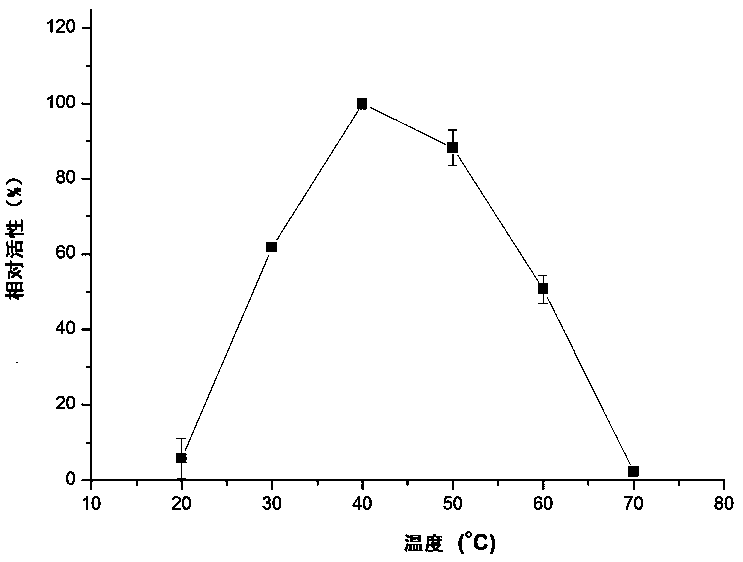
Laccase gene lblcc9i from Ceruleus bicolor and its application
A technology for decolorization of laccase and dye, applied in the field of laccase LbLCC9I sequence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 LbLCC9I Gene expression in Pichia pastoris
[0026] 1. Construction of Pichia pastoris expression vector
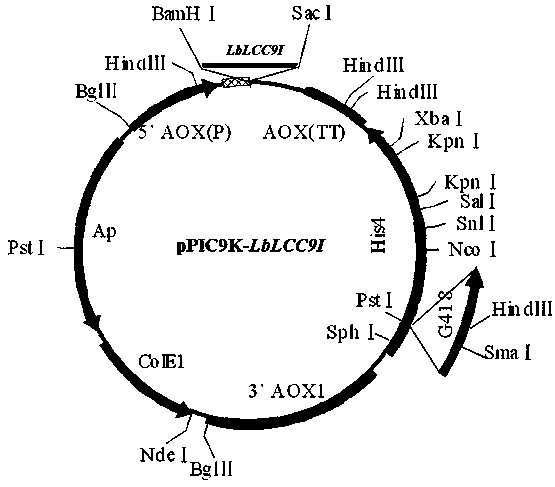
[0027] The optimized gene sequence is shown as SEQ ID NO 1, and the whole gene synthesis is carried out. The gene fragment was ligated to a T / A cloning vector (Takara). Transform DH5α competent to obtain positive clones. The positive clone plasmid was extracted, and the Bam H1 and Sac 1 After double digestion, insert the correct reading frame into the expression vector pPIC9K of Pichia pastoris, and verify it by sequencing to obtain the recombinant plasmid pPIC9K- LbLCC9I (See Figure 2).
[0028] 2. Transformation of Pichia pastoris
[0029] The GS115 (mut + his - , invitrogen) monoclonal inoculation into 10 mL YPD, cultured overnight at 30°C, 225 rpm, transferred 1 mL to 100 mL fresh YPD and continued to culture for 3-5 h to OD 600 =0.5, 5000 rpm centrifuged to collect the bacterial cells. After washing twice with an equal volume of double...
Embodiment 2
[0039] Example 2 LbLCC9I Gene expression in Arabidopsis
[0040] one, LbLCC9I Construction of Gene Plant Expression Vector
[0041] The optimized gene fragment was ligated with T / A cloning vector (Takara). Transform DH5α competent to obtain positive clones. The positive clone plasmid was extracted, and the Bam H1 and Sac 1 After double digestion, insert the correct reading frame into the plant expression vector pYPX158, and verify it by sequencing to obtain the recombinant plasmid pYPX158- LbLCC9I (See Figure 6).
[0042] 2. Transformation of Arabidopsis
[0043] pYPX158- LbLCC9I Transform Agrobacterium tumefaciens GV3101, or EHA105, or LBA4404 strains (Biovector Co., LTD) by electric shock method to obtain pYPX158- LbLCC9I The recombinant Agrobacterium was spread on a plate containing kanamycin resistance to select transformants. The above transformants were inoculated in YEB liquid medium (containing 100 μg / ml kanamycin and 75 μg / ml rifampicin), and cultured...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com