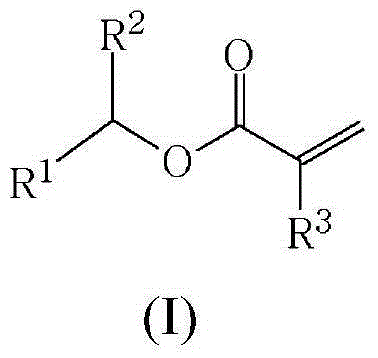
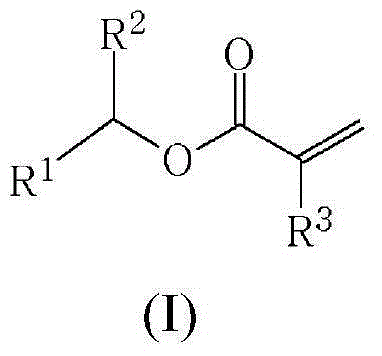
Method for preparing (meth)acrylates of biobased alcohols and polymers thereof
A secondary alkyl acrylate, bio-based technology, applied in the field of dehydration of bio-based alcohols
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0228] These examples are for illustration purposes only, and are not intended to unduly limit the scope of the appended claims. Notwithstanding that the numerical ranges and parameters setting forth the broad scope of the disclosure are approximations, the numerical values set forth in the specific examples are reported as precisely as possible. Any numerical value, however, inherently contains certain errors necessarily resulting from the standard deviation found in their respective testing measurements. At the very least, and not as an attempt to limit the application of the doctrine of equivalents to the scope of the claims, each numerical parameter should at least be construed in light of the number of reported significant digits and by applying ordinary rounding techniques.
[0229] material overview
[0230] All parts, percentages, ratios, etc. in the examples, as well as in the remainder of the specification, are by weight unless otherwise indicated. Table 1 prov...
example 1
[0240] Example 1: Octene derived from 1-octanol
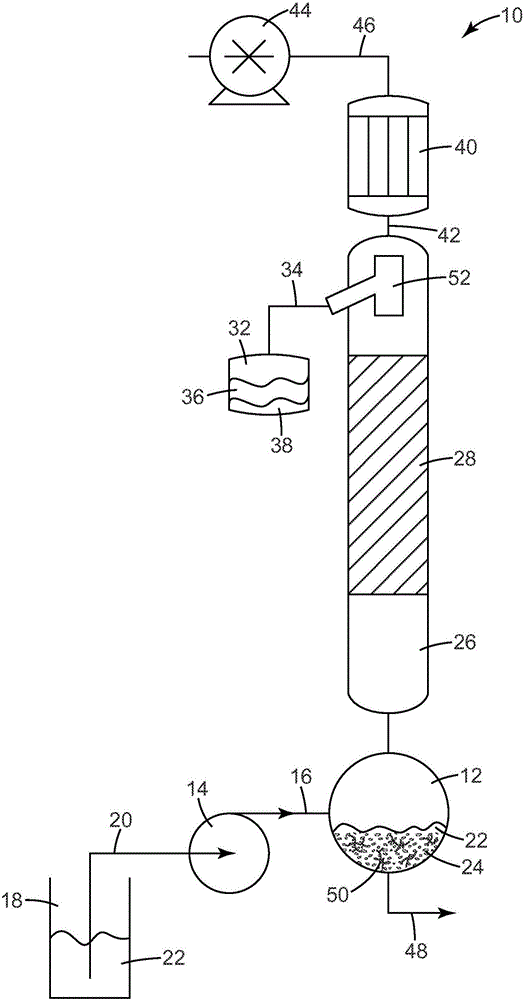
[0241] A 2 liter (L) Erlenmeyer flask was charged with 1200 grams (g) of 1-octanol derived from vegetable oil and 100 g of AMBERLY ST70 catalyst material (sulfonated styrene divinylbenzene copolymer). The flask was heated to about 180°C and heated at 4.2 ml / min (mLmin -1 ) at a rate of 1-octanol was continuously fed into the flask. During the reaction, product water and octene evaporate into a 1.5 inch (3.8 cm) internal diameter (I.D.) by 24 inch (61 cm) long fractionation column equipped with a condenser and reflux ratio controller (i.e., reflux valve), which were kept constant at 5°C and a 1:1 ratio, respectively. After at least 1 hour of continuous operation at 2.51gh -1 (g catalyst) -1 (6.97×10 -4 gs -1 (g catalyst) -1 ) rate was collected for analysis and found to consist primarily of a mixture of octene isomers and water. Water was then separated from the product mixture using a separatory funnel to yield the oc...
example 2
[0242] Example 2: Octene derived from 2-octanol
[0243] A 3 L round bottom flask was charged with 800 g of 2-octanol derived from vegetable oil and 100 g of AMBERLY ST70 catalyst material (sulfonated styrene divinylbenzene copolymer). The flask was heated to about 130°C and heated at 6.66mLmin -1 The rate of 2-octanol was continuously fed into the flask. During the reaction, the product water and octene evaporate into a 1.5 inch (3.8 cm) ID x 24 inch (61 cm) long splitter column equipped with a condenser and reflux ratio controller (i.e., reflux valve) , which are kept constant at 5°C and a 1:1 ratio, respectively. After at least 1 hour of continuous operation, at 4.716gh -1 (g catalyst) -1 (1.31×10 -3 gs -1 (g catalyst) -1 ) rate was collected for analysis and found to consist primarily of a mixture of octene isomers and water. Water was then separated from the product mixture using a separatory funnel to yield the octene product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com