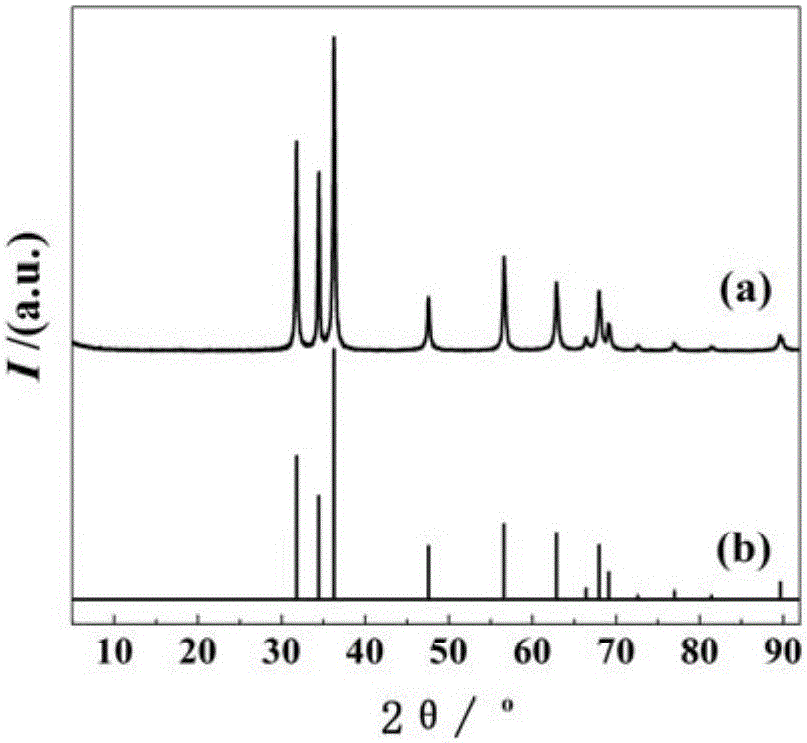
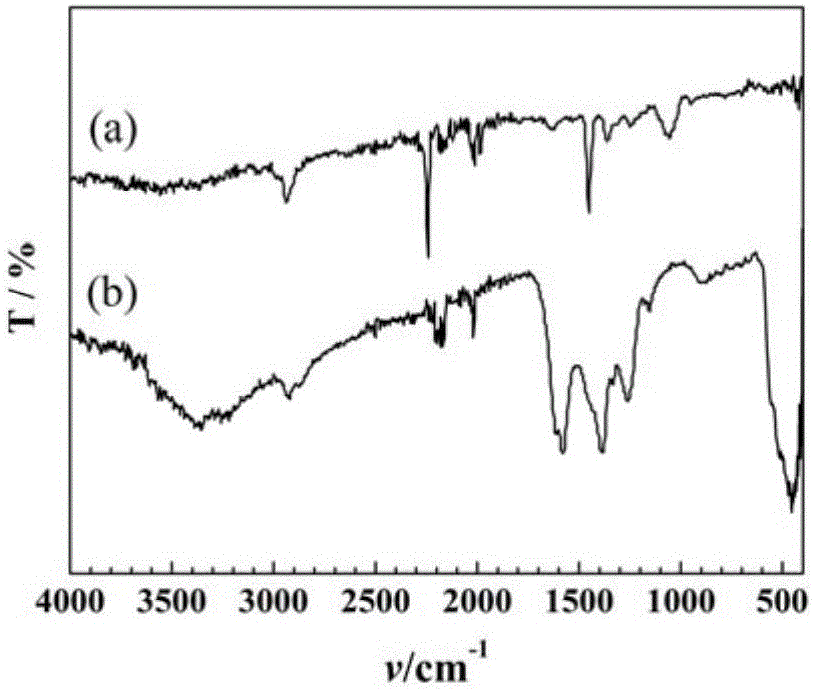
Preparation method of nano zinc oxide/cyclized polyacrylonitrile composite micro-sphere material with zinc oxide nano particles uniformly distributed in polymer
A zinc oxide nano and nano zinc oxide technology, applied in organic compound/hydride/coordination complex catalysts, chemical instruments and methods, chemical/physical processes, etc. The chain rigidity is large, and the number of lamellae is difficult to control effectively, so as to achieve the effect of excellent visible light catalytic activity, simple preparation method and improved absorption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Weigh 1.00 g of polyacrylonitrile solid and dissolve it completely in dimethyl sulfoxide to make 50 mL of polyacrylonitrile dimethyl sulfoxide solution, and take another 2.00 g of zinc nitrate hexahydrate solid and dissolve it in 5.0 mL of dimethyl sulfoxide Then, 27.0 mL of the polyacrylonitrile dimethyl sulfoxide solution was pipetted and mixed with the zinc nitrate dimethyl sulfoxide solution to obtain a mixed solution of zinc nitrate and polyacrylonitrile dimethyl sulfoxide. Then dilute 2.20 g of an ammonia solution with an ammonia concentration of 25% in 320 mL of distilled water to prepare a dilute ammonia solution with a mass concentration of 0.17%. Then, under rapid stirring conditions, the mixed solution of zinc nitrate and polyacrylonitrile in dimethyl sulfoxide was slowly added dropwise to the dilute ammonia solution for 30 minutes, and the stirring was continued for 30 minutes after the addition was completed, and then left to stand After 12 hours, the produ...
Embodiment 2
[0034] Weigh 1.00g of polyacrylonitrile solid and dissolve it in 50mL dimethyl sulfoxide solvent, take another 2.00g of zinc nitrate hexahydrate solid and dissolve it in 5.0mL dimethyl sulfoxide solvent, then pipette polyacrylonitrile dimethyl sulfoxide 9.0 mL of the sulfone solution was airtightly mixed with the above zinc nitrate dimethyl sulfoxide solution to obtain a mixed solution of zinc nitrate and polyacrylonitrile dimethyl sulfoxide. Then dilute 2.20 g of an ammonia solution with an ammonia concentration of 25% in 140 mL of distilled water to prepare a dilute ammonia solution with a mass concentration of 0.39%. Then slowly add the mixed solution of zinc nitrate and polyacrylonitrile dimethyl sulfoxide into the dilute ammonia solution under the condition of rapid stirring, the dropping time is 20min, and continue to stir for 30min after the dropping to make it fully react After standing still for 12 hours, the product was filtered, washed three times with water, and dr...
Embodiment 3
[0036] Weigh 1.00 g of polyacrylonitrile solid and dissolve it completely in dimethyl sulfoxide to make 50 mL of polyacrylonitrile dimethyl sulfoxide solution, and take another 2.00 g of zinc nitrate hexahydrate solid and dissolve it in 5.0 mL of dimethyl sulfoxide Then, 27.0 mL of the polyacrylonitrile dimethyl sulfoxide solution was pipetted and mixed with the zinc nitrate dimethyl sulfoxide solution to obtain a mixed solution of zinc nitrate and polyacrylonitrile dimethyl sulfoxide. Then dilute 2.20 g of an ammonia solution with an ammonia concentration of 25% in 320 mL of distilled water to prepare a dilute ammonia solution with a mass concentration of 0.17%. Then, under the condition of rapid stirring, the mixed solution of zinc nitrate and polyacrylonitrile in dimethyl sulfoxide was slowly added dropwise to the dilute ammonia solution for 25 minutes, and the stirring was continued for 30 minutes after the addition was completed, and then left to stand After 10 hours, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com