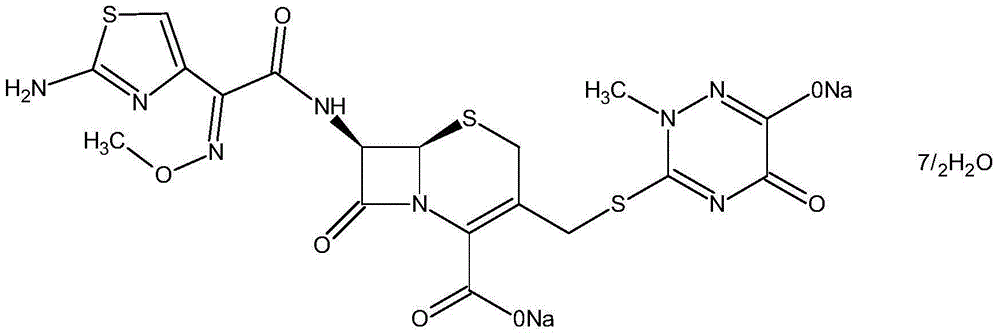
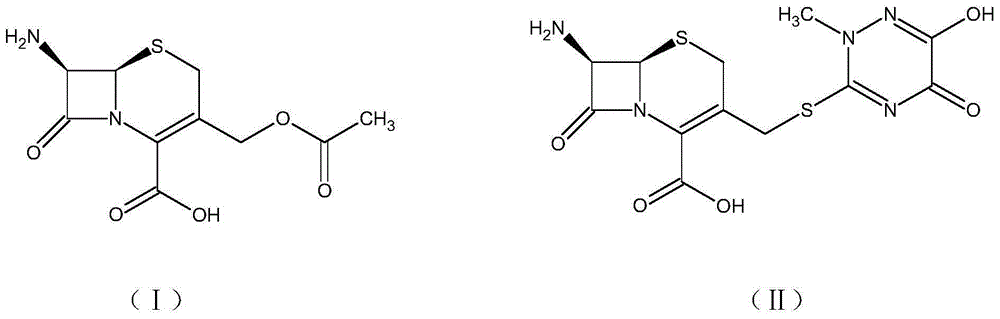
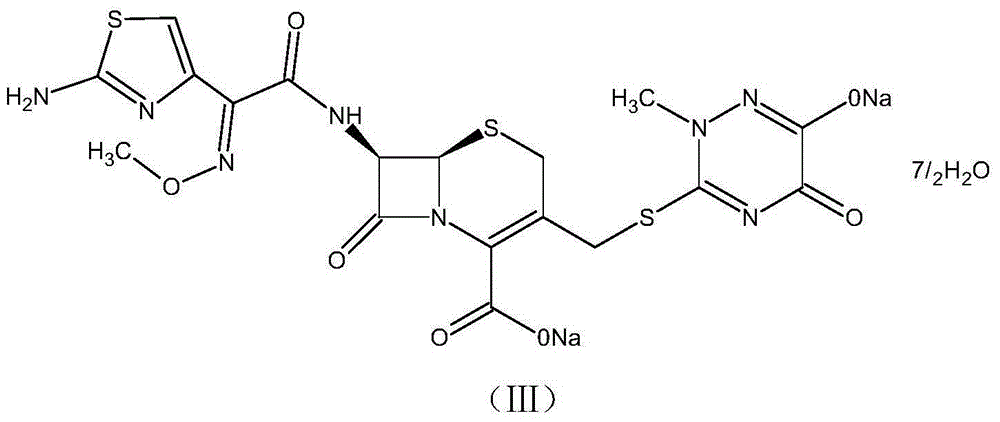
One-pot synthesis method of ceftriaxone sodium
A technology of ceftriaxone sodium and a synthesis method, which is applied in the field of medicinal chemical synthesis, can solve the problems of drug contamination, physical injury of operators, troublesome operation, etc., and achieves the effects of avoiding potential pollution, simplifying production operations, and increasing production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Control the temperature at 20-25°C, put 35g of boron trifluoride ethyl acetate complex into 150ml of ethyl acetate, stir until it is completely dissolved, add 15g of triazine ring, 25g of 7-ACA, and control the temperature at 30°C for timing React for 20 minutes, add 120ml of water after the reaction, immediately lower the temperature to 5°C, stir, let stand to separate layers, and wash the organic phase twice with 120ml of water to obtain the ethyl acetate phase of 7-ACT;
[0051] (2) Add 100ml of dichloromethane, 30ml of methanol, 1ml of sulfurous acid, and 15ml of water to the ethyl acetate phase of 7-ACT in sequence, then add 31g of AE active ester, control the temperature at 2°C, and add 25ml of triethylamine dropwise for 45min After the internal dropwise addition is completed, keep warm until the reaction solution residue is detected to be qualified, add 23g of sodium acetate and 100g of purified water for salt formation and extraction, stand for stratification...
Embodiment 2
[0053] (1) Control the temperature at 20-25°C, put 35g of boron trifluoride ethyl acetate complex into 150ml of ethyl acetate, stir until it is completely dissolved, add 15g of triazine ring, 25g of 7-ACA, and control the temperature at 30°C for timing React for 20 minutes, add 120ml of water after the reaction, immediately lower the temperature to 6°C, stir, let stand to separate layers, and wash the organic phase twice with 120ml of water to obtain the ethyl acetate phase of 7-ACT;
[0054] (2) Add 100ml of dichloromethane, 30ml of ethanol, 0.35g of sodium metabisulfite, 15ml of water to the ethyl acetate phase of 7-ACT, then add 31g of AE active ester, control the temperature at 1°C, and add 18.5ml of diethylamine dropwise , the dropwise addition is completed within 60 minutes, keep warm until the residual of the reaction solution is qualified, add 23g of sodium acetate and 100g of purified water for salt formation and extraction, stand for stratification, decolorize and fil...
Embodiment 3
[0056] (1) Control the temperature at 20-25°C, put 35g of boron trifluoride ethyl acetate complex into 150ml of ethyl acetate, stir until completely dissolved, add 15g of triazine ring, 25g of 7-ACA, and control the temperature at 28°C for timing React for 20 minutes, add 120ml of water after the reaction, immediately lower the temperature to 8°C, stir, let stand to separate layers, and wash the organic phase twice with 120ml of water to obtain the ethyl acetate phase of 7-ACT;
[0057] (2) Add 100ml of dichloromethane, 30ml of isopropanol, 0.30g of sodium thiosulfate, and 15ml of water to the ethyl acetate phase of 7-ACT in sequence, then add 31g of AE active ester, control the temperature at 3°C, and drop 22.5 ml of tetramethylguanidine, the dropwise addition is completed within 60 minutes, keep the reaction until the residual of the reaction solution is qualified, add 14g of sodium bicarbonate and 100g of purified water for salt formation and extraction, stand for stratifica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com