Synthesis method of carbapenem antibiotic parent nucleus MAP
A technology for carbapenems and a synthesis method, which is applied in the field of pharmaceutical intermediates, can solve the problems of inability to meet the requirements of environmental protection, many three wastes, etc., and achieves the effects of less harsh reaction conditions, low price, and reduced energy consumption and cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
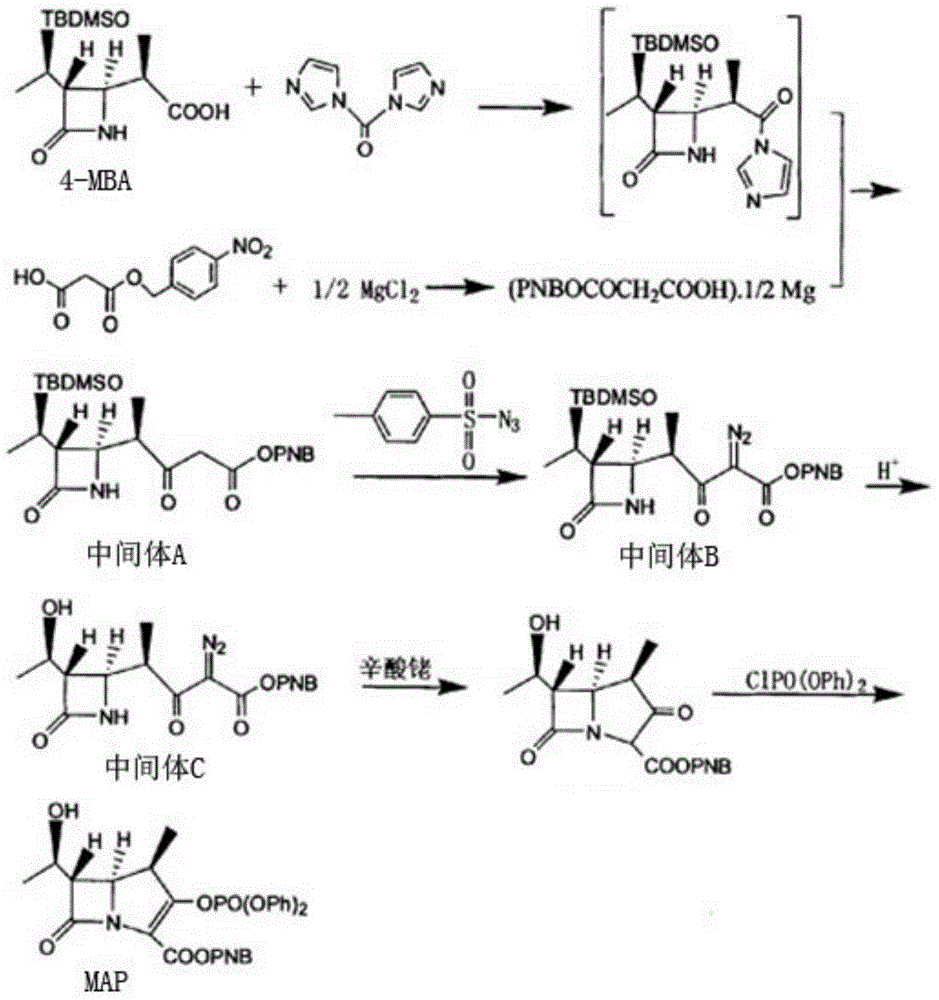
[0025] A kind of synthetic method of carbapenem antibiotic mother nucleus MAP is characterized in that, comprises the following steps:
[0026] 1) Add p-nitrobenzyl malonate, dichloromethane, and anhydrous magnesium chloride into a nitrogen-protected reaction vessel, and add triethylamine dropwise at room temperature, and react for 3 hours to obtain anhydrous magnesium salt;
[0027] 2) Add 4-MBA, dichloromethane, CDI and anhydrous magnesium salt into a nitrogen-protected reaction vessel, keep it warm at 30°C for 7-8 hours, add hydrochloric acid after cooling down to 25°C, stir for 30 minutes, let stand to separate and separate Separate the organic layer to obtain the organic solution of intermediate A;
[0028] 3) Add toluenesulfonyl azide to the organic solution of intermediate A, raise the temperature to 50°C for 2 hours, concentrate at 40°C to obtain a brown oil, then add isopropanol to the brown oil, and cool down to Crystallization at 5-10°C, and solid-liquid separation...
Embodiment 2
[0036] A kind of synthetic method of carbapenem antibiotic mother nucleus MAP is characterized in that, comprises the following steps:
[0037] 1) Add p-nitrobenzyl malonate, dichloromethane, and anhydrous magnesium chloride into a nitrogen-protected reaction vessel, and add triethylamine dropwise at room temperature, and react for 2 hours to obtain anhydrous magnesium salt;
[0038] 2) Add 4-MBA, dichloromethane, CDI and anhydrous magnesium salt into a nitrogen-protected reaction vessel, keep warm at 20°C for 7h, add hydrochloric acid after cooling down to 20°C, stir for 20min, let stand to separate layers and separate the organic Layer obtains the organic solution of intermediate A;
[0039] 3) Add toluenesulfonyl azide to the organic solution of intermediate A, raise the temperature to 50°C for 1 hour, concentrate at 40°C to obtain a brown oil, then add isopropanol to the brown oil, cool down to Crystallization was carried out at 5°C, and intermediate B was obtained by sol...
Embodiment 3
[0047] A kind of synthetic method of carbapenem antibiotic mother nucleus MAP is characterized in that, comprises the following steps:
[0048]1) Add p-nitrobenzyl malonate, dichloromethane, and anhydrous magnesium chloride into a nitrogen-protected reaction vessel, and add triethylamine dropwise at room temperature, and react for 3 hours to obtain anhydrous magnesium salt;
[0049] 2) Add 4-MBA, dichloromethane, CDI and anhydrous magnesium salt into a nitrogen-protected reaction vessel, keep warm at 30°C for 8h, add hydrochloric acid after cooling down to 30°C, stir for 30min, let stand to separate layers and separate the organic Layer obtains the organic solution of intermediate A;
[0050] 3) Add toluenesulfonyl azide to the organic solution of intermediate A, heat up to 50°C for 2 hours, concentrate at 45°C to obtain a brownish-yellow oil, then add isopropanol to the brownish-yellow oil, cool down to Crystallization was carried out at 10°C, and intermediate B was obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com