Method for rapidly generating dense silver chloride plating layer, and silver chloride electrode prepared through method
A silver chloride, dense technology, applied in the field of corrosion and protection of reinforced concrete structures, can solve the problems of general AgCl electrode reproducibility, tedious preparation of anodic chlorination, large difference in response curve slope, etc., to achieve potential reproducibility Excellent, reduced manufacturing time, improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
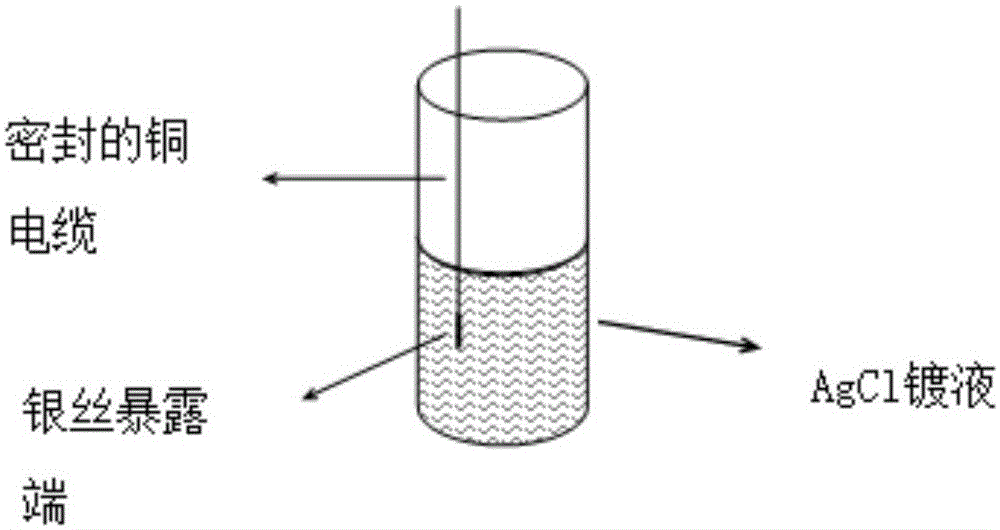
[0032] Prepare the chloride ion selective electrode: weld one end of the silver wire to one end of the copper cable, seal the welding place with epoxy resin, then pretreat the silver wire at the exposed end according to the above method, soak the pretreated silver wire in AgCl In the plating solution: the immersion time in the AgCl plating solution and the sodium hypochlorite solution content in the AgCl plating solution are shown in Table 1 below.
[0033] AgCl plating solution comprises the following components by weight percentage: sodium hypochlorite solution (wherein available chlorine content 10%) content is shown in Table 1, and solid caustic soda content is 1%, and fatty alcohol polyoxyethylene ether sulfate sodium salt (AES) content is 0.35% , and the balance is distilled water.
[0034] Wherein, the silver wire has a diameter of 0.3 mm and a length of 8 mm. The welding end of the silver wire, the welding place with the copper cable, and the welding end of the copper...
Embodiment 2
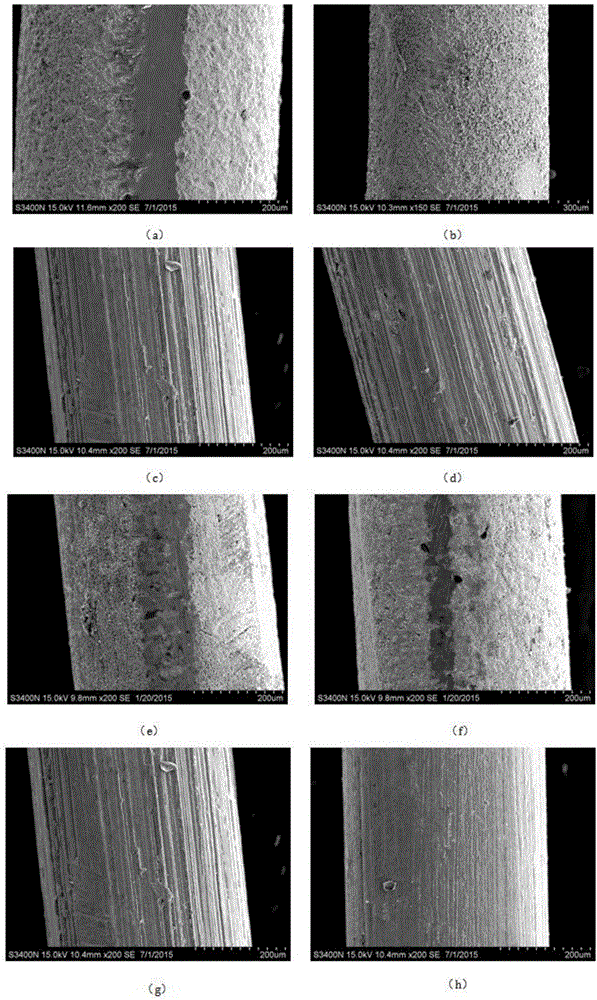
[0039] Such as figure 2 as shown, figure 2 The SEM picture of the electrode prepared for Example 1; for the chloride ion selective electrode produced in Example 1, the surface microscopic morphology of the prepared electrode was observed with an electron microscope. figure 2 (a)-(h) in (a)-(h) respectively correspond to the SEM figure of the electrode of A1-A8 that embodiment 1 prepares because A3 and A7 preparation method are the same, so figure 2 (c) and figure 2 (g) is the same. From figure 2 From (a) to 2(d), it can be seen that when the immersion time is the same, the higher the sodium hypochlorite content in the plating solution composition, the denser the electrode surface coating. In addition, when the sodium hypochlorite content reaches 40%, the coating on the electrode surface is very dense, even if the concentration of sodium hypochlorite is increased again, the compactness of the coating will not increase significantly; by figure 2 From (e) to 2(h), it ...
Embodiment 3
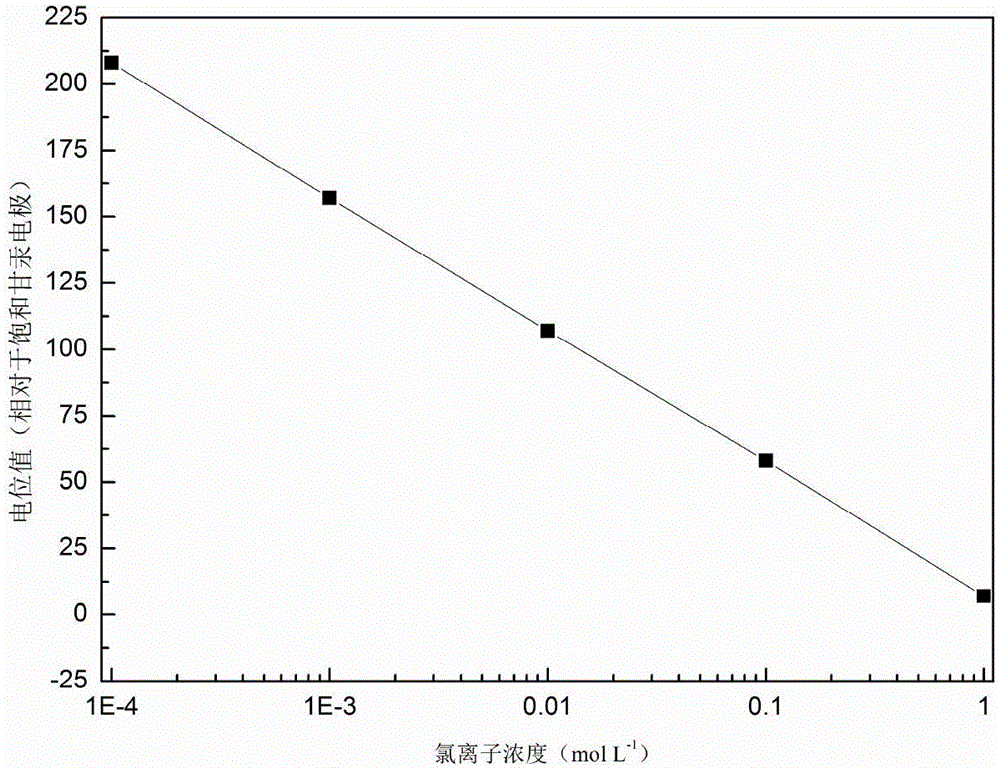
[0041] Select the electrode whose serial number is A3 among the embodiment 1, and the chloride ion content is respectively 1,0.1,0.01,10 -3 and 10 -4 moL -1 The electrode potential was tested in the concrete simulated liquid.
[0042] According to the Nernst formula, the electrode potential can be expressed by the following formula (1):
[0043]
[0044] However, the response relationship between the actual electrode potential and the log value of the chloride ion concentration cannot strictly follow the Nernst formula, so it is necessary to determine the true slope of the electrode potential to the log value of the chloride ion concentration. Such as image 3As shown, the slope of the response curve of the electrode potential and the log value of the chloride ion concentration is about 50mV / decade.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com