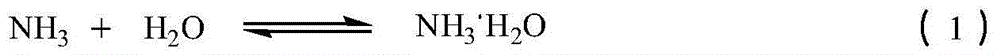Method for producing ammonium sulfamate by utilization of exhaust gas containing ammonia and carbon dioxide
A technology of ammonium sulfamate and carbon dioxide, which is applied in the direction of sulfamic acid, nitrogen and non-metallic compounds, can solve the problems of complex process, low economic value, cumbersome operation, etc., and achieve the goal of simple process, reduced consumption and reduced energy consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
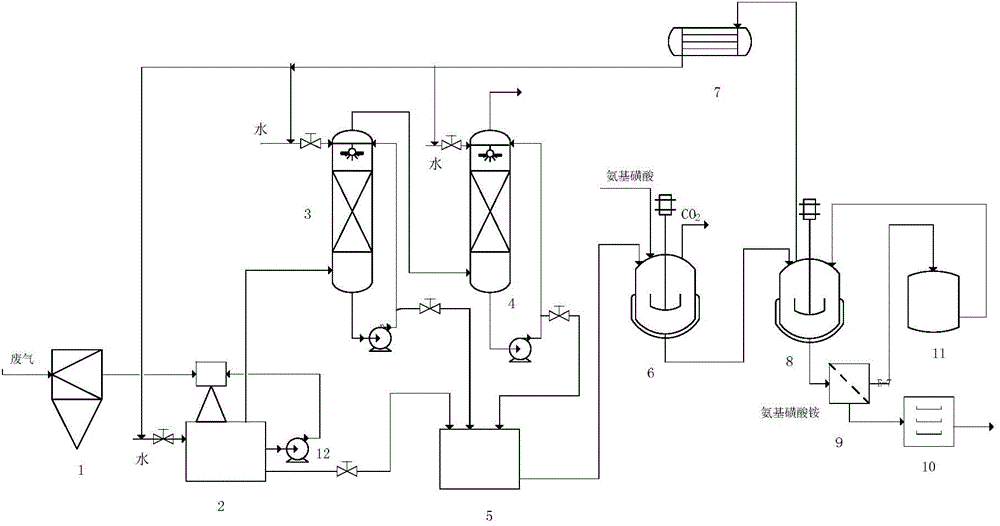
[0044] Embodiment 1 uses water to absorb the waste gas produced in the sodium cyanate process by the urea method.
[0045] The amount and composition of high-temperature waste gas produced by the industrial production of 1 ton of sodium cyanate by urea method are shown in Table 1.1.
[0046] Table 1.1 The amount and composition of waste gas produced by urea process industrial production of 1 ton of sodium cyanate
[0047] composition
[0048] water
[0049] After the above-mentioned waste gas passes through the cyclone separator to remove solid particles, it enters the water jet vacuum pump, and then passes into the two-stage absorption tower with absorption liquid circulation. Used 1110Kg water to absorb waste gas altogether (production 1 ton of sodium cyanate product), obtain the absorption liquid 2020Kg that contains ammonia and carbon dioxide in the absorption liquid tank. The specific gravity of the absorption liquid is 1.15Kg / L, the temperature is 1...
Embodiment 2
[0050] Embodiment 2 uses the exhaust gas absorption liquid containing ammonia and carbon dioxide to prepare ammonium sulfamate
[0051] The absorption liquid (specific gravity is 1.15Kg / L in 500Kg embodiment 1, and temperature is 16 ℃, contains ammonia 16.6%, contains carbon dioxide 19.9%) in the synthetic kettle, and adds 470Kg ammonium sulfamate in the synthetic kettle, control The feeding speed is controlled, and the temperature in the kettle is controlled in the range of 0-60° C., and the reaction is carried out until the pH in the kettle is = 7. 98.5Kg of carbon dioxide was obtained. At the same time, all the reaction solution was put into the precipitation kettle, and vacuum distilled at 80°C. Distill until the specific gravity of the solution is 1.40Kg / L, cool to 10°C, crystallize, and filter. Obtain 300K filtrate, 200Kg filter cake. The filtrate is sent to the mother liquor tank for the next batch to be applied mechanically, and mixed with the reaction liquid of the...
Embodiment 3
[0052] Embodiment 3 uses the exhaust gas absorption liquid containing ammonia and carbon dioxide to prepare ammonium sulfamate
[0053] The absorption liquid (specific gravity is 1.15Kg / L in 500Kg embodiment 1, and temperature is 16 ℃, contains ammonia 16.6%, contains carbon dioxide 19.9%) in the synthetic kettle, and adds 470Kg ammonium sulfamate in the synthetic kettle, control The feeding speed is controlled, and the temperature in the kettle is controlled in the range of 0-60° C., and the reaction is carried out until the pH in the kettle is = 7. 98.4Kg of carbon dioxide was obtained. At the same time, all the reaction solution was added into the precipitation kettle, and the 300K filtrate obtained in Example 2 was added into the precipitation kettle from the mother liquor tank. Vacuum distillation at 80°C. Distill until the specific gravity of the solution is 1.43Kg / L, cool to 10°C, crystallize, and filter. Obtain 300K filtrate, 557Kg filter cake. The filtrate is sent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com