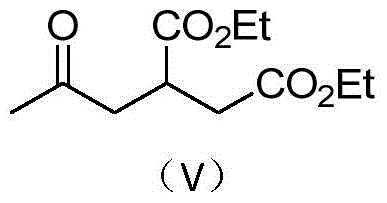
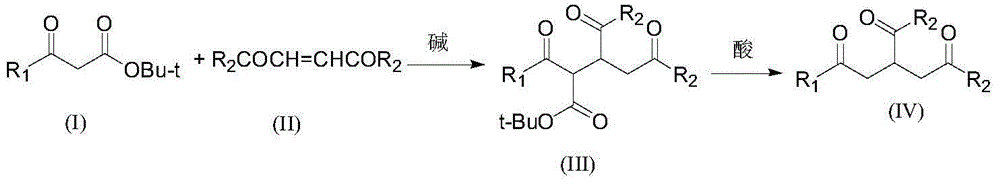
A kind of preparation method and its intermediate of 2-alkanoylmethyl-1,4-butanedioic acid derivative
A technology of alkanoylmethyl and succinic acid, which is applied in the field of preparation of 2-alkanoylmethyl-1,4-butanedioic acid derivatives, can solve problems such as safety risks, and achieve short reaction time and high reaction selection Good sex and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of 2-acetylmethyl-1,4-diethyl succinate
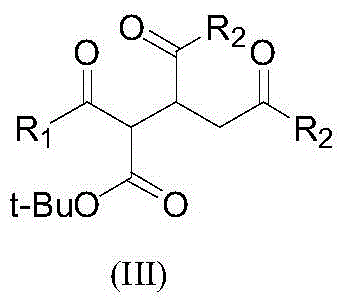
[0026] Step 1 Add 200 g of tert-butyl acetoacetate and 320 g of diethyl maleate to a 1 L three-neck equipped with a mechanical stirrer, a thermometer and a drying tube. 1.42 g of potassium tert-butoxide was added with stirring. After the addition, continue stirring for 5 h, add 400 mL of chloroform, 100 g of water and 7.06 g of industrial hydrochloric acid, and separate the organic phase. After precipitation of the organic phase, 410 g of diethyl 2-[(acetyl)(tert-butoxycarbonyl)]methyl-1,4-butanedioate was obtained. MS: m / z=330.1 ([M] + ).
[0027] Step 2 Add 408 g of diethyl 2-[(acetyl)(tert-butoxycarbonyl)]methyl-1,4-butanedioate, 1000 mL of toluene, and 21.6 g of p-toluenesulfonic acid into the reaction flask, and react for 2 h. After washing successively with 200 mL of water and 200 mL of 5% sodium bicarbonate solution, the solution was desolvated under reduced pressure to obtain 256 g of diethyl 2...
Embodiment 2
[0028] Example 2: Preparation of 2-benzoylmethyl-1,4-diethyl succinate
[0029] Step 1 Add 277 g of tert-butyl benzoylacetate and 320 g of diethyl maleate to a 1 L three-neck equipped with a mechanical stirrer, a thermometer and a drying tube. 14 g of potassium tert-butoxide was added with stirring. After the addition is complete, continue stirring for 4 h, add 400 mL of chloroform, 100 g of water and 7.06 g of industrial hydrochloric acid, and separate the organic phase. After precipitation of the organic phase, 490 g of diethyl 2-[(benzoyl)(tert-butoxycarbonyl)]methyl-1,4-butanedioate was obtained.
[0030] Step 2 Add 488 g of diethyl 2-[(acetyl)(tert-butoxycarbonyl)]methyl-1,4-butanedioate, 1,000 mL of toluene, and 21.6 g of p-toluenesulfonic acid into the reaction flask, and react for 3 hours. After washing successively with 200 mL of water and 200 mL of 5% sodium bicarbonate solution, the solution was desolvated under reduced pressure to obtain 300 g of diethyl 2-benzoy...
Embodiment 3
[0031] Example 3: Preparation of N-ethyl-2-acetylmethyl-1,4-succinimide
[0032] Step 1 Add 158 g of tert-butyl acetoacetate, 125 g of N-ethylmaleimide, and 500 mL of ethanol into a 1 L three-neck equipped with a mechanical stirrer, a thermometer and a drying tube. 7.8 g of sodium ethoxide was added with stirring. After the addition, the stirring reaction was continued for 8h. After desolvation under reduced pressure, 200 mL of chloroform, 100 g of water and 11.8 g of industrial hydrochloric acid were added to separate the organic phase, and the product after the desolvation of the organic phase was directly put into the next step.
[0033] Step 2: Add the product obtained in Step 1 into the reaction flask, add 600 mL of n-hexane and 13.7 g of p-toluenesulfonic acid, and react for 3 hours. Cool down, wash with 200mL of water and 150mL of 5% sodium bicarbonate solution successively, and precipitate under reduced pressure to obtain 165g of the product with a yield of 84%. MS:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com