Preparation method of trinexapac-ethyl and intermediate thereof
An intermediate, trinexapac-ethyl technology, which is applied in the field of preparation of trinexapac-ethyl and its intermediates, can solve the problems of affecting the quality of trinexapac-ethyl, increasing the cost of purification, and low reaction efficiency, so as to avoid high-temperature and high-pressure reactions and by-products Less, good reaction selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
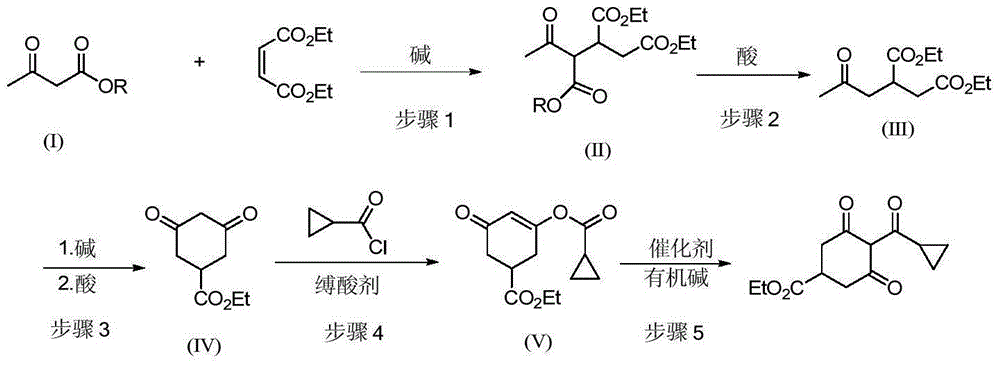
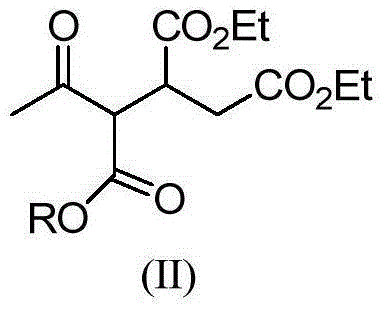
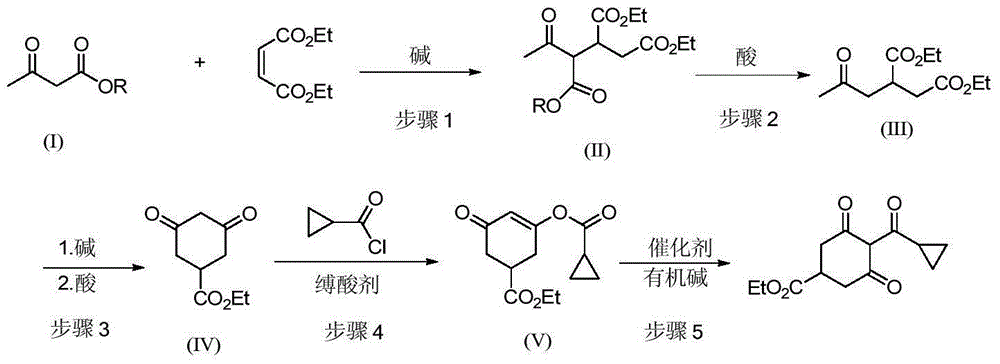
[0028] 1) Preparation of ethyl 4-oxopentane-1,2-dicarboxylate-3-tert-butyl carboxylate
[0029] Add 200 g of tert-butyl acetoacetate and 228 g of diethyl maleate to a 1 L three-neck equipped with a mechanical stirrer, a thermometer and a drying tube. 1.4 g of potassium tert-butoxide were added with stirring. After the addition, the stirring reaction was continued for 5h. Add 400 mL of chloroform, 100 g of water and 7.06 g of industrial hydrochloric acid, separate the layers, and desolventize the organic phase to obtain 410 g of ethyl 4-oxopentane-1,2-dicarboxylate-3-tert-butyl carboxylate. MS: m / z = 330.1 ([M+1]+).
[0030] 2) Preparation of 2-acetonyl-1,4-diethyl succinate
[0031] Add 410g of 4-oxopentane-1,2-dicarboxylate ethyl-3-tert-butylcarboxylate to the reaction flask, add 1000mL of toluene, 21.6g of p-toluenesulfonic acid, and stir for 2h. After successively washing with 200 mL of water and 200 mL of 5% sodium bicarbonate solution, 256 g of diethyl 2-acetonyl-1,4-...
Embodiment 2
[0039] 1) Preparation of ethyl 4-oxopentane-1,2-dicarboxylate-3-isopropyl carboxylate
[0040] Add 137 g of isopropyl acetoacetate and 172 g of diethyl maleate to a 1 L three-neck equipped with a mechanical stirrer, thermometer and drying tube. 3 g of potassium hydroxide were added with stirring. After the addition, the stirring reaction was continued for 5h. Add 300 mL of chloroform, 100 g of water and 5 g of industrial hydrochloric acid, separate the layers, and desolventize the organic phase to obtain 306 g of ethyl 4-oxopentane-1,2-dicarboxylate-3-isopropyl carboxylate.
[0041] 2) Preparation of 2-acetonyl-1,4-diethyl succinate
[0042] Add 306g of ethyl 4-oxopentane-1,2-dicarboxylate-3-isopropyl carboxylate to the reaction flask, add 800mL of toluene and 15.3g of p-toluenesulfonic acid, and stir for 2h. After successively washing with 150 mL of water and 150 mL of 5% sodium bicarbonate solution, 200 g of diethyl 2-acetonyl-1,4-butanedioate was obtained by precipitatio...
Embodiment 3
[0050] 1) Preparation of ethyl 4-oxopentane-1,2-dicarboxylate-3-isopropyl carboxylate
[0051] Add 165 g of isopropyl acetoacetate and 206 g of diethyl maleate to a 1 L three-neck equipped with a mechanical stirrer, a thermometer and a drying tube. 6.5 g of potassium hydroxide was added with stirring. After the addition, the stirring reaction was continued for 6h. Add 800mL of toluene, 200g of water and 6.3g of industrial hydrochloric acid, separate the layers, and the organic phase containing the crude product is directly used in the next reaction.
[0052] 2) Preparation of 2-acetonyl-1,4-diethyl succinate
[0053] Add the above-mentioned toluene solution and 12 g of trifluoroacetic acid into the reaction flask, stir for 2 h, wash with 200 mL of water and 200 mL of 5% sodium bicarbonate solution in turn, and desolvate under reduced pressure to obtain 230 g of 2-acetonyl-1,4-butanedioic acid diethyl ester.
[0054] 3) Preparation of ethyl 3,5-cyclohexanedione-1-carboxylat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com