A high-yield l-amino acid oxidase strain and its application
An amino acid and oxidase technology, which is applied in the direction of enzymes, bacteria, and microorganism-based methods, can solve problems such as harsh reaction conditions, large equipment corrosion, and complicated separation, and achieve stable product quality, energy saving in the process, and good biological safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] In this example:
[0041] 1) The complete medium consists of glucose 2 g / L, peptone 10 g / L, yeast powder 5 g / L, NaCl 5 g / L, pH 7.0, solid medium plus agar 15 g / L;
[0042] 2) The basic medium consists of glucose 20 g / L, sodium citrate 5 g / L, K 2 HPO 4 7 g / L, KH 2 PO 4 7 g / L, MgSO 4 0.1 g / L, (NH 4 ) 2 SO 4 2 g / L, pH 7.0, solid medium plus agar 15 g / L;
[0043] 3) The screening medium is 40 μg / mL lysine added to the basic medium;
[0044]4) LB slant medium / seed medium is peptone 10 g / L, yeast powder 5 g / L, NaCl 5 g / L, pH7.0, solid medium plus agar 15 g / L;
[0045] 5) The fermentation medium is peptone 50g / L, (NH 4 ) 2 SO 4 5.5g / L 、NaH 2 PO 4 2H 2 O 4.2 g / L, K 2 HPO 4 ·3H 2 O 8.7 g / L, (NH 4 ) 2 SO 4 5.5 g / L, MgSO 4 ·7H 2 O 2.5 g / L or MgSO 4 3.7 g / L, ammonia water and 2 mol / L hydrochloric acid were used to control the pH of the fermentation broth during the fermentation process.
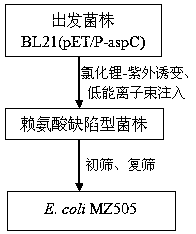
[0046] Such as figure 1 As shown, the specific steps for obt...
Embodiment 2
[0060] This example illustrates the physiological and biochemical characteristics of Escherichia coli MZ505
[0061] The morphological and physiological and biochemical characteristics of Escherichia coli MZ505 obtained in the above-mentioned embodiment 1 are as follows:
[0062] Colony color: milky white to yellowish.
[0063] Bacteria morphology: round colony with metallic luster.
[0065] Colony size: 0.2-0.8 x 1-4 μm.
[0066] Aerobic mode: aerobic growth.
[0067] Suitable growth temperature: about 37 ℃.
[0068] Suitable growth pH: around 7.0.
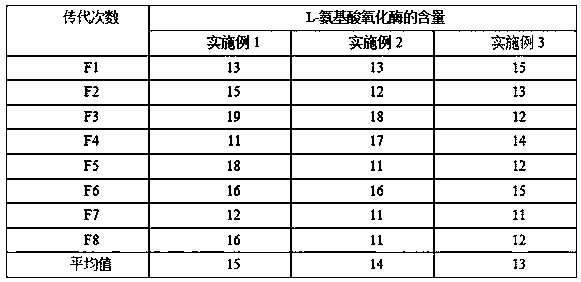
[0069] The genetic stability test of Escherichia coli MZ505 in Example 1, and the strain subculture and fermentation experiments are shown in Table 1.
[0070] Table 1 is the genetic stability test of Escherichia coli MZ505
[0071]
[0072] It can be seen from experiments that after 8 passages, the content of L-amino acid oxidase in the genetically engineered bacteria is stable, and has g...
Embodiment 3
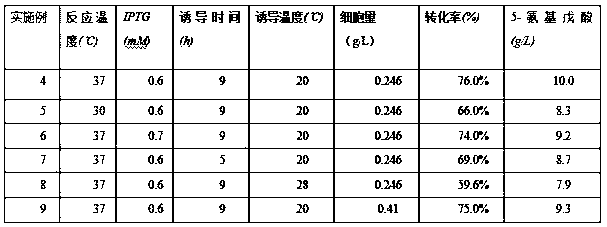
[0074] The activated seed liquid after the overnight culture of the embodiment was inserted into the shake flask culture medium, and the culture conditions were as follows: temperature 37 ° C, stirring speed 200 rpm, inoculum size was 1% by volume, Kana Resistance is 0.2% by volume. When the cell mass of the strain grew to 0.246g / L, 0.6 mM IPTG was added to induce expression at 20°C, and cultured at a stirring speed of 200 rpm for 9 h; the crude enzyme solution of L-amino acid oxidase was obtained by ultrasonic crushing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com