Preparation method of mesoporous ferric hydroxide adsorbent used for adsorbing highly toxic pollutant Cr(VI)
A mesoporous hydroxyl and adsorbent technology, applied in chemical instruments and methods, adsorbed water/sewage treatment, other chemical processes, etc., can solve the problems of cumbersome process, low utilization rate of raw materials, difficult control of reaction conditions, etc., and achieve the preparation method. Simple, fast adsorption kinetics, cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
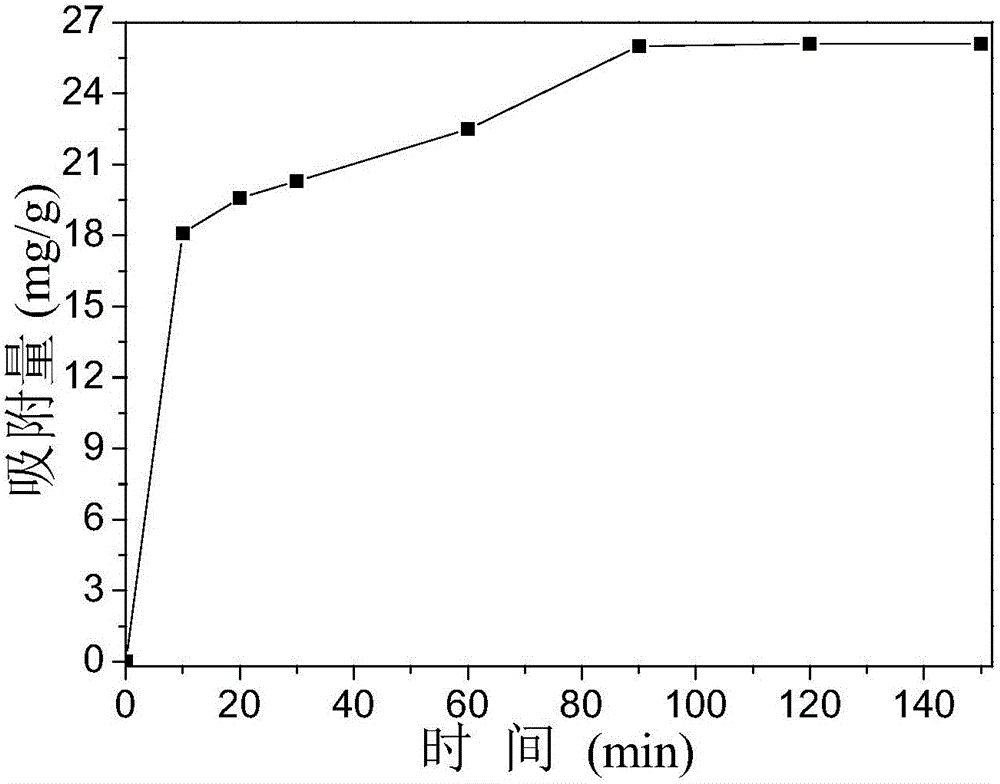
[0031] 8.14gFe(NO 3 ) 3 9H 2 O was dissolved in a small amount of distilled water and made to volume in a 100mL volumetric flask to obtain solution A; 4.02g of urea was dissolved in a small amount of distilled water and made to volume in a 100mL volumetric flask to obtain solution B; 2.0g of P123 was added to 40mL of absolute ethanol , and stirred for 30 min to obtain solution C. Then, solution C was mixed with solution A and solution B in a beaker respectively (Fe in the mixed solution 3+ The concentration is 0.084mol / L, the concentration of urea is 0.279mol / L, the concentration of P123 is 0.025mol / L), after stirring evenly, it is transferred to a drying oven at 85°C for heating for 15h. After the reaction, the reaction product cooled to room temperature was centrifuged, and the precipitate was isolated for the first time, and distilled water was added to wash and separate (the centrifuge speed was set at 5000r / min), and the operation was repeated twice; then dehydrated al...
Embodiment 2
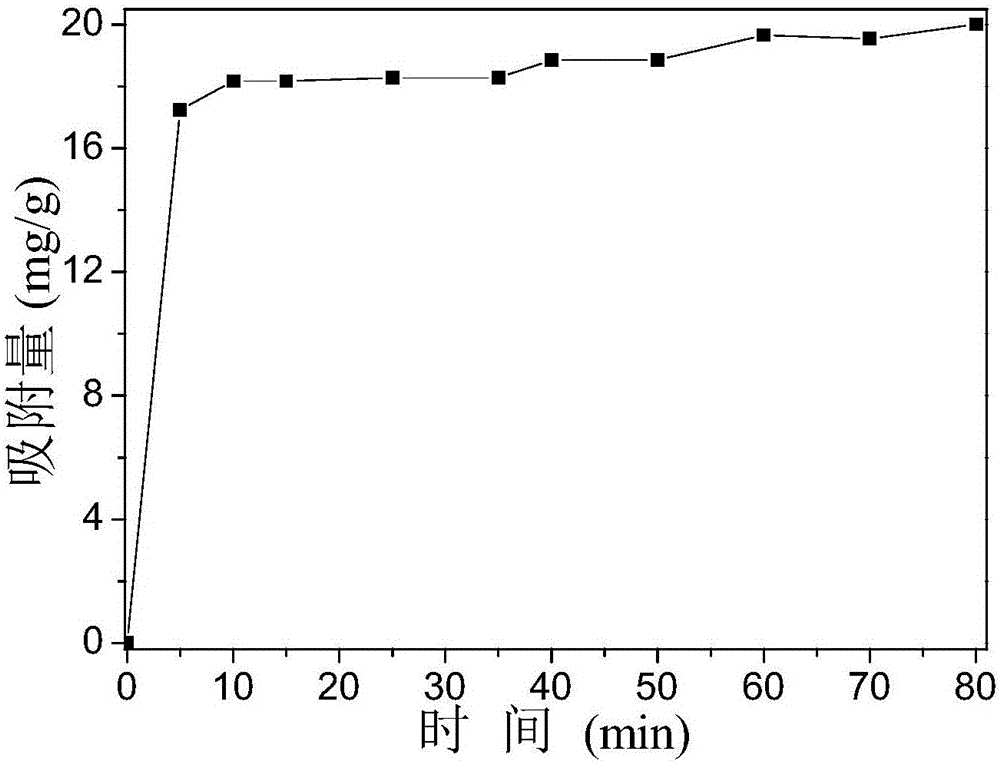
[0034] 4.0394gFe 2 (SO 4 ) 3 Dissolve in a small amount of distilled water and dilute to volume in a 100mL volumetric flask to obtain solution A; dissolve 4.0502g urea in a small amount of distilled water and dilute to volume in a 100mL volumetric flask to obtain solution B; add 2.26g of P123 to 40mL of absolute ethanol, Stir for 30 min to obtain solution C. Then, solution C was mixed with solution A and solution B in a beaker respectively (Fe in the mixed solution 3+The concentration is 0.008mol / L, the concentration of urea is 0.281mol / L, the concentration of P123 is 0.0283mol / L), after stirring evenly, transfer it to a drying oven at 80°C for heating for 16h. After the reaction, the reaction product cooled to room temperature was centrifuged, the precipitate was isolated for the first time, and distilled water was added to wash and separate (the speed of the centrifuge was set at 5000r / min), and the operation was repeated twice; Set to 7000r / min), repeat the operation th...
Embodiment 3
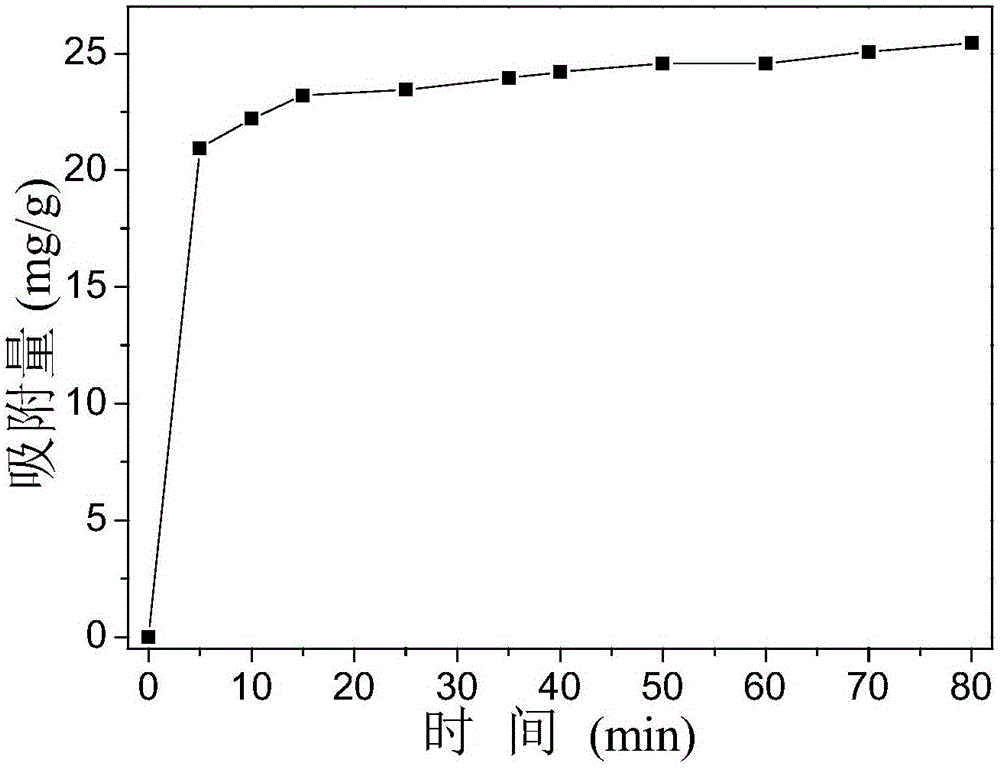
[0037] 0.65gFeCl 3 Dissolve in a small amount of distilled water and make up to volume in a 100mL volumetric flask to obtain solution A; dissolve 6.04g of urea in a small amount of distilled water and make up to volume in a 100mL volumetric flask to obtain solution B. Add 2.3g of P123 to 40ml of absolute ethanol and stir for 30min to obtain solution C. Then, solution C was mixed with solution A and solution B in a beaker respectively (Fe in the mixed solution 3+ The concentration is 0.0167mol / L, the concentration of urea is 0.419mol / L, the concentration of P123 is 0.0288mol / L), after stirring evenly, transfer it to a drying oven at 100°C for heating for 3h. After the reaction, the reaction product cooled to room temperature was centrifuged, the precipitate was isolated for the first time, and distilled water was added to wash and separate (the speed of the centrifuge was set at 5000r / min), and the operation was repeated twice; Set to 7000r / min), repeat the operation three ti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com