Method for preparing long-chain alkane from biomass derivativeS 5-HMF (hydroxymethyl furfural) or furaldehyde
A technology of 5-HMF and long-chain alkanes, which is applied in the field of preparation of long-chain alkanes, can solve the problems of poor product selectivity and achieve the effects of less catalyst consumption, clean process and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of condensation product methyl-5-hydroxymethyl furan vinyl ketone
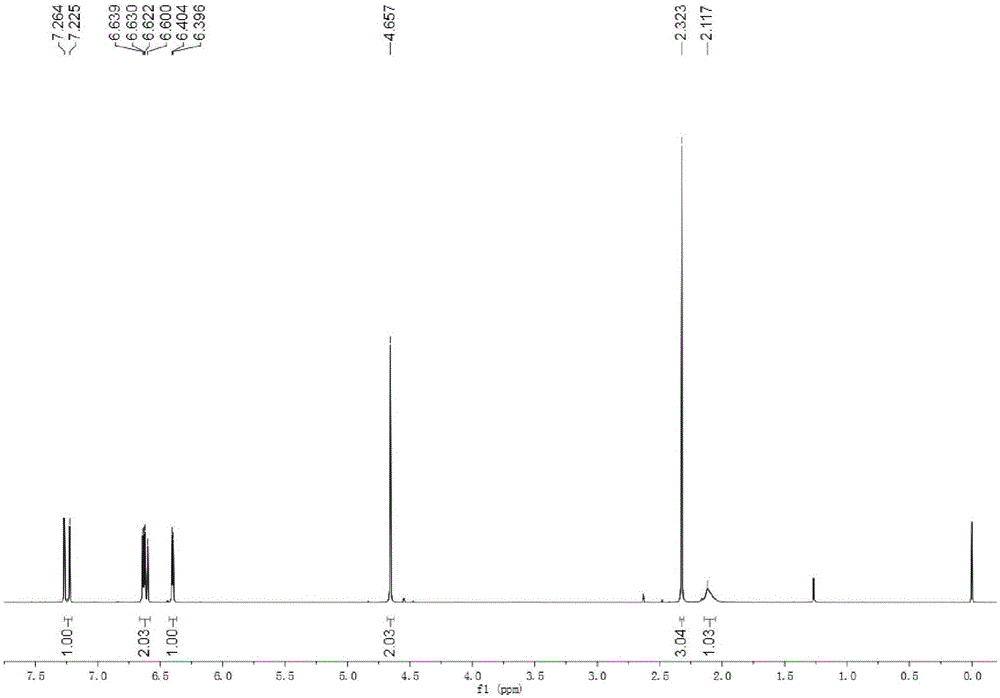
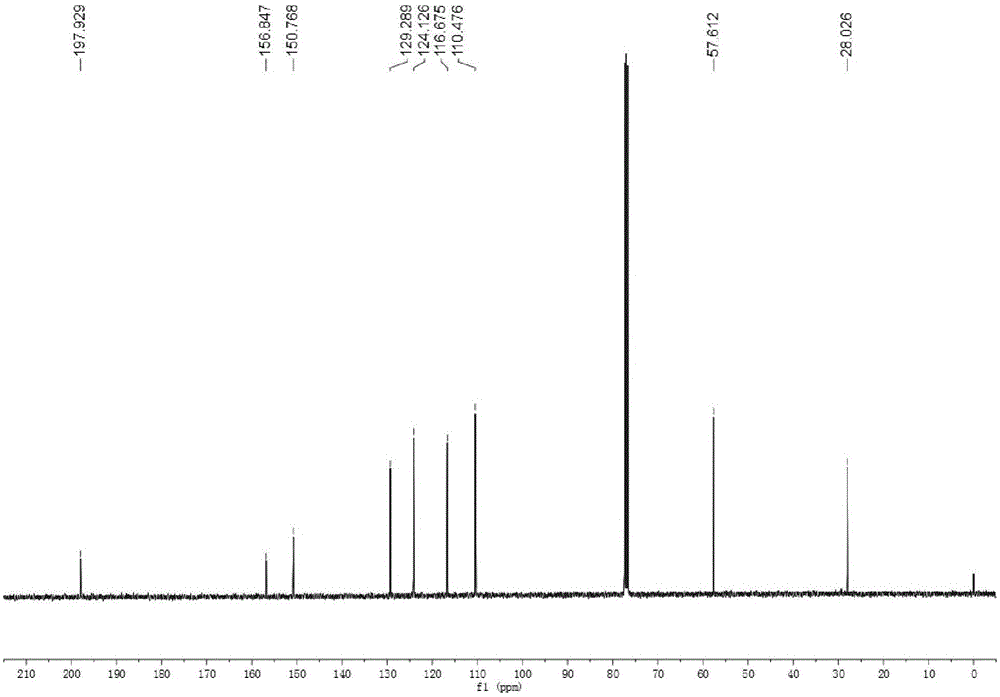
[0029] Add 10g of 5-HMF into a 500mL round-bottomed flask, then add 150mL of acetone to it, stir and dissolve at room temperature. After the 5-HMF is completely dissolved, add 100 mg of solid NaOH powder into the flask, and stir at room temperature for 10 h; after the reaction is completed, filter, dry over anhydrous magnesium sulfate, and filter again, spin the liquid to dry, dissolve the sample in ethyl acetate, Column chromatography separation (eluent is sherwood oil: ethyl acetate=1:1, volume ratio), obtains 8.3g condensation product methyl-5-hydroxymethylfuran vinyl ketone (NMR sees figure 1 , 2 ).
Embodiment 2
[0030] Embodiment 2: the preparation of condensation product methyl furan vinyl ketone
[0031]Add 10g of furfural into a 500mL round bottom flask, then add 150mL of acetone to it, and stir evenly at room temperature. Then add 200mg of MgO powder to the flask, and stir at room temperature for 20h; after the reaction is over, filter, dry over night over anhydrous magnesium sulfate, and filter again, spin the liquid to dry, dissolve the sample in ethyl acetate, and separate by column chromatography (the eluent is Petroleum ether:ethyl acetate=1:1, volume ratio), to obtain 7.2g of condensation product methyl furan vinyl ketone.
Embodiment 3
[0032] Embodiment 3: the preparation of condensation product isobutyl-5-hydroxymethyl furan vinyl ketone
[0033] Add 10g of 5-HMF into a 500mL round bottom flask, then add 100mL of methyl isobutyl ketone into it, and stir to dissolve at room temperature. After the 5-HMF is completely dissolved, add 200mgAl to the flask 2 o 3 powder, stirred at room temperature for 20h; after the reaction was completed, filter, and dry overnight over anhydrous magnesium sulfate, and after filtering again, the liquid was spin-dried, dissolved in ethyl acetate and mixed, and separated by column chromatography (the eluent was petroleum ether: ethyl acetate =1:1, volume ratio), to obtain 7.5g of condensation product isobutyl-5-hydroxymethyl furfuryl ketone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com