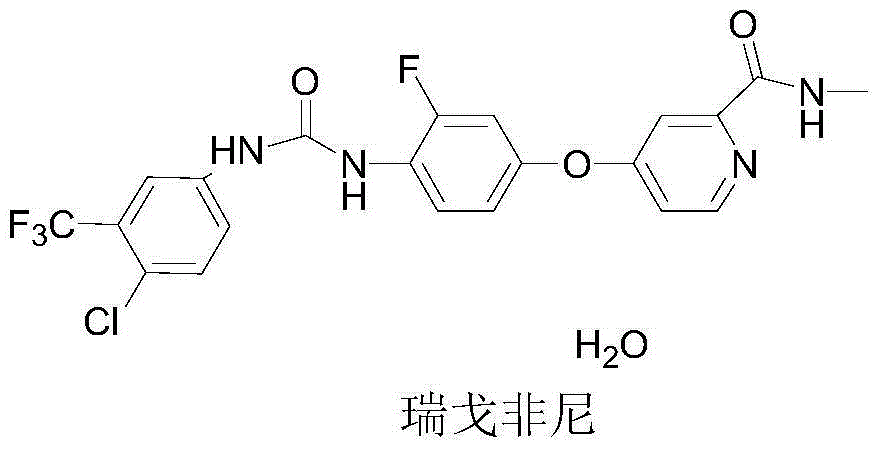
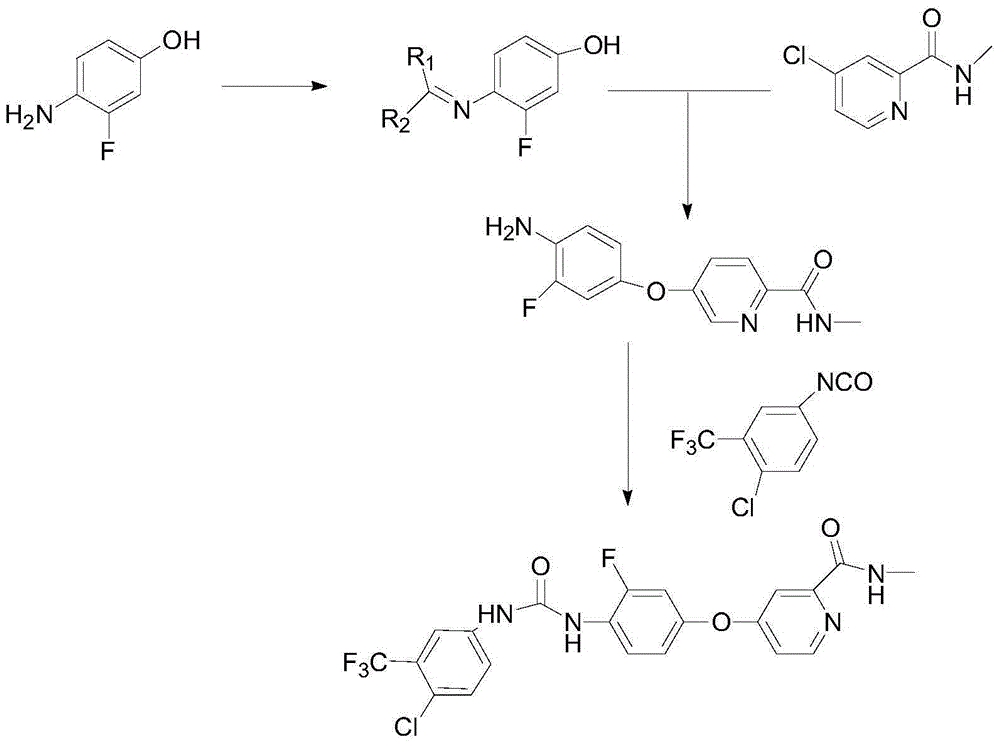
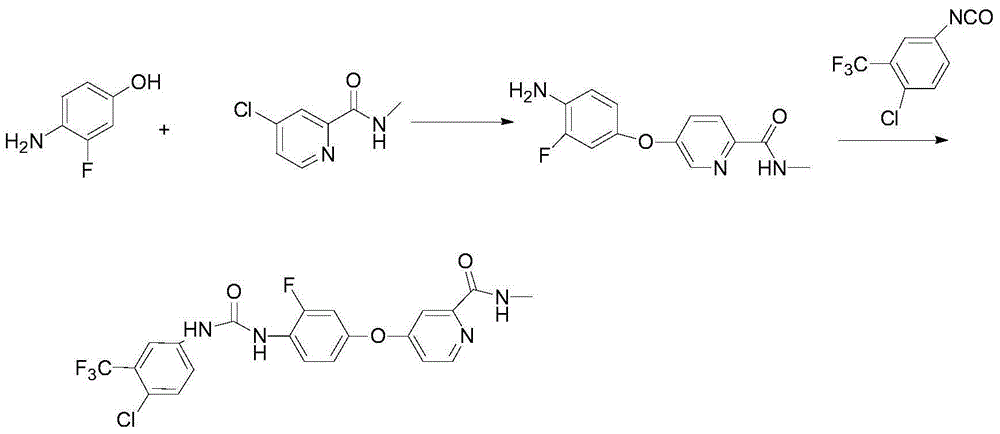
Regorafenib preparation method
A technology of regorafenib and compounds, which is applied in the field of preparation of regorafenib, can solve the problems of difficulty in realizing industrial production, expensive raw and auxiliary materials, and low total yield, and achieve short reaction time, easy control, and total yield. The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The first step, the preparation of compound IV: add 127.1g of 4-amino-3-fluorophenol and 1L of dichloromethane in a 2L reaction flask, add 202.4g of triethylamine and dropwise add 327.8g of di-tert-butyl dicarbonate under stirring After 5 hours of reaction at room temperature, TLC detected that the reaction of the raw materials was complete, and the reaction was stopped. The system was washed with 3×500mL of 5% sodium bicarbonate and then washed with 3×500mL of water. The organic layer was dried and filtered, and the solvent was evaporated to obtain 200.2g of a white solid. Yield: 88.1%, HPLC: 98.7%.
[0036] In the second step, compound II is first prepared, and the specific steps are as follows:
[0037] Add 171.6g of methyl 4-chloropyridine-2-carboxylate, 200mL of methanol and 500mL of tetrahydrofuran solution into a 3L reaction flask, cool the system down to 0°C, and add 1030mL of tetrahydrofuran solution containing 85.8g of methylamine dropwise after the temperatur...
Embodiment 2
[0046] The first step, the preparation of compound IV: add 127.1g of 4-amino-3-fluorophenol and 1L of dichloromethane into a 2L reaction flask, add 202.4g of triethylamine and 418.2g of triphenylchloromethane under stirring, and complete the addition. After 7 hours of reaction at room temperature, TLC detection showed that the reaction of the raw materials was complete, and the reaction was stopped. The system was washed with 5% sodium bicarbonate 3X500mL and then washed with 3X500mL water. The organic layer was dried and filtered, and the solvent was evaporated to obtain 203.9g of a white solid. %, HPLC: 98.7%.
[0047] The reaction conditions of the second step and the third step are the same as in Example 1.
Embodiment 3
[0049] Preparation of Compound III: Add 45.4 g of Compound IV (R is Boc) and 300 mL of DMA to a reaction flask of Compound 3L, add 28.8 g of sodium tert-butoxide under stirring, stir at room temperature for 1 hour, then add 34.1 g of Compound II, after the addition is complete, the temperature of the system is raised to 60 React at ℃ for 9 hours, HPLC detects that the reaction is complete, slowly pour the system into 1L ethyl acetate, after the addition is complete, stir for 10 minutes, wash with 3X800mL water, extract the water layer with 1L ethyl acetate, combine the organic layers, and use anhydrous magnesium sulfate for the organic layer After drying, filter, evaporate the solvent to obtain a brown oil, add 1L of methanol to the system, add 5mol / L hydrochloric acid solution, stir at room temperature for 2h and filter, add 500mL of water and 1L of ethyl acetate to the filter cake, and simultaneously use 5mol / L The sodium hydroxide solution was neutralized to adjust the pH to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com