Chitosan-alkoxy formamide and preparation method thereof
A technology of alkoxy formamide and chitosan, which is applied in the field of chitosan-alkoxy formamide and its preparation, can solve the problems of irregular and different structures, and achieve high structural regularity, chemical characteristics or physical properties. Good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Preparation of chitosan:
[0033] Pulverize the purchased flake chitin (shrimp shell) with a pulverizer, sieve (355 μm), collect the chitin under the sieve as raw material, and use two methods to deacetylate chitin (refer to literature 10-12);
[0034] The embodiment of the present invention adopts gel chromatography to measure the number average molecular weight (M) of chitosan n), when measuring the molecular weight of chitosan, the acetic acid (0.3M)-ammonium acetate (0.1M) buffer solution was used as the mobile phase, and polyethylene glycol was used as the standard.
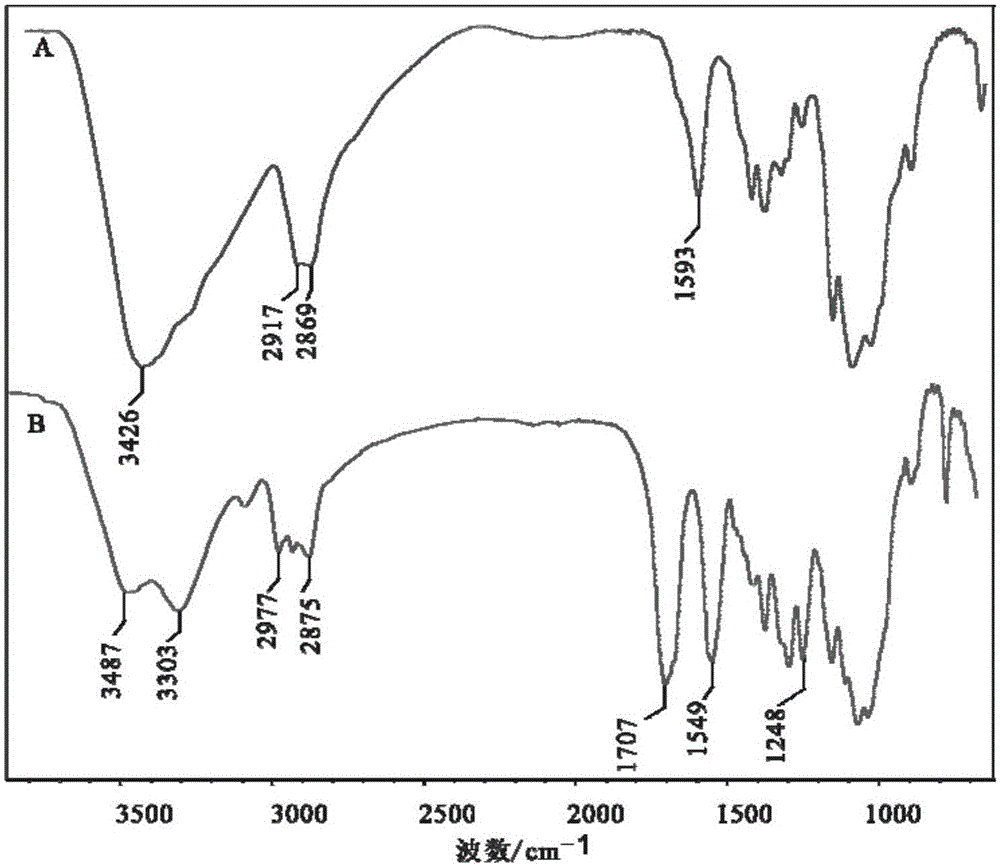
[0035] Both chitosan and N-acylated chitosan can adsorb water molecules, so the existence of adsorbed water molecules should be considered when calculating element values.
Embodiment 1
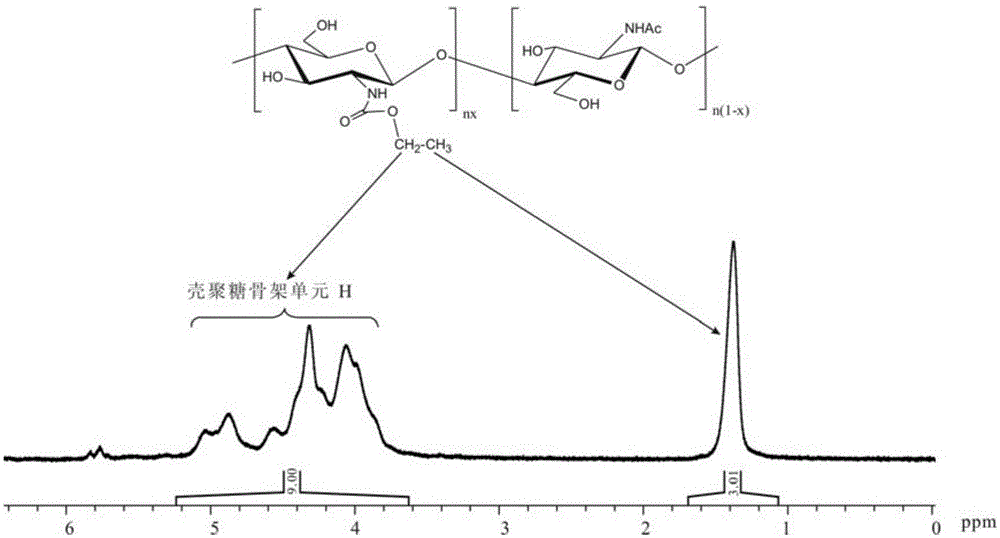
[0037] Synthesis of chitosan-ethoxy formamide
[0038] 1) Add 1.50g (9.32mmol repeating unit) chitosan (100,000 number average molecular weight, 100% deacetylation degree) into a 250mL three-necked flask, add 20.00g of prepared 1.2wt% dilute hydrochloric acid solution, stir Dissolve chitosan until clear and transparent. Then weigh 25.00 g of methanol, add about 20.00 g of methanol into the three-necked flask, mechanically stir it evenly, and place the three-necked flask in an ice-water bath (temperature about 4° C.). After the temperature in the solution dropped and stabilized, 2.01 g (18.64 mmol) of ethyl chloroformate was quickly added. After reacting at 4°C for about 15 minutes, weigh 1.88g (18.64mmol) of triethylamine (the molar ratio of triethylamine to ethyl chloroformate is 1:1) and mix it with the remaining methanol, and add an appropriate amount of triethylamine Adjust the pH of the reaction solution to about 6, and intermittently add triethylamine during the reacti...
Embodiment 2
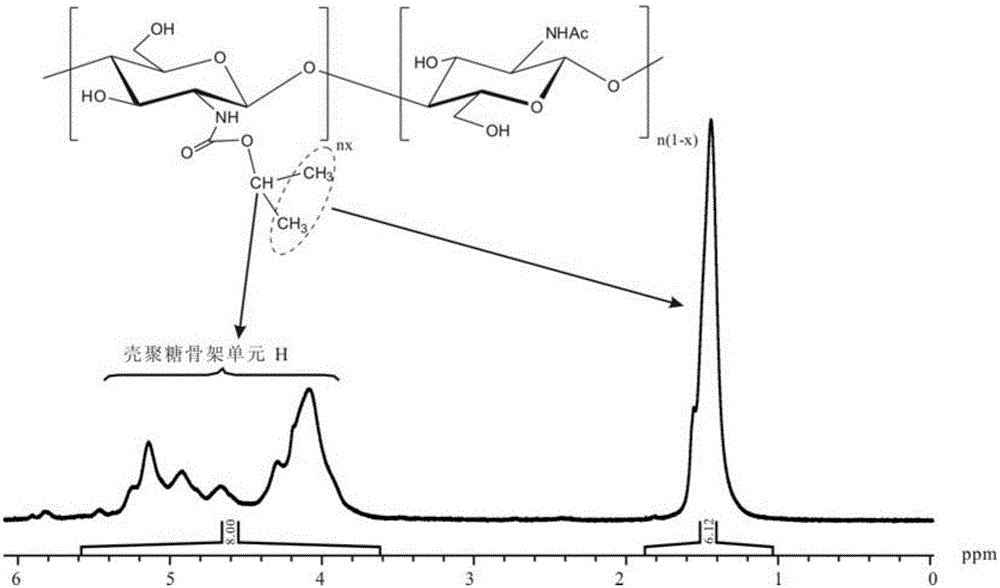
[0041] Synthesis of Chitosan-n-Pentoxyformamide
[0042] 1) 1.40g (8.69mmol repeating unit) chitosan (350,000 number average molecular weight, 98% degree of deacetylation) was added to a 250mL three-necked flask, and 36.95g of prepared 0.87wt% dilute hydrochloric acid solution was added, stirred Dissolve chitosan until clear and transparent. Then weigh 36.08g of methanol, add about 90% methanol into the three-necked flask, mechanically stir the three-necked flask to an ice-water bath (temperature about 6° C.). After the temperature in the solution dropped and stabilized, 10.18 g (69.52 mmol) of n-pentyl chloroformate was quickly added. Stir for 30min and mix well, remove the ice-water bath to allow the reaction to proceed at 26-27°C, weigh 7.02g (69.52mmol) of triethylamine (the molar ratio of triethylamine to n-pentyl chloroformate is 1:1) and Mix the remaining methanol, add an appropriate amount of triethylamine to adjust the pH of the reaction solution to about 6, and add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com