A kind of preparation method of polyester-polycarbonate-polyester multi-block copolymer
A multi-block copolymer and polycarbonate technology, applied in the field of polymer materials, can solve problems such as narrow dispersion, and achieve the effect of simple operation and simple structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
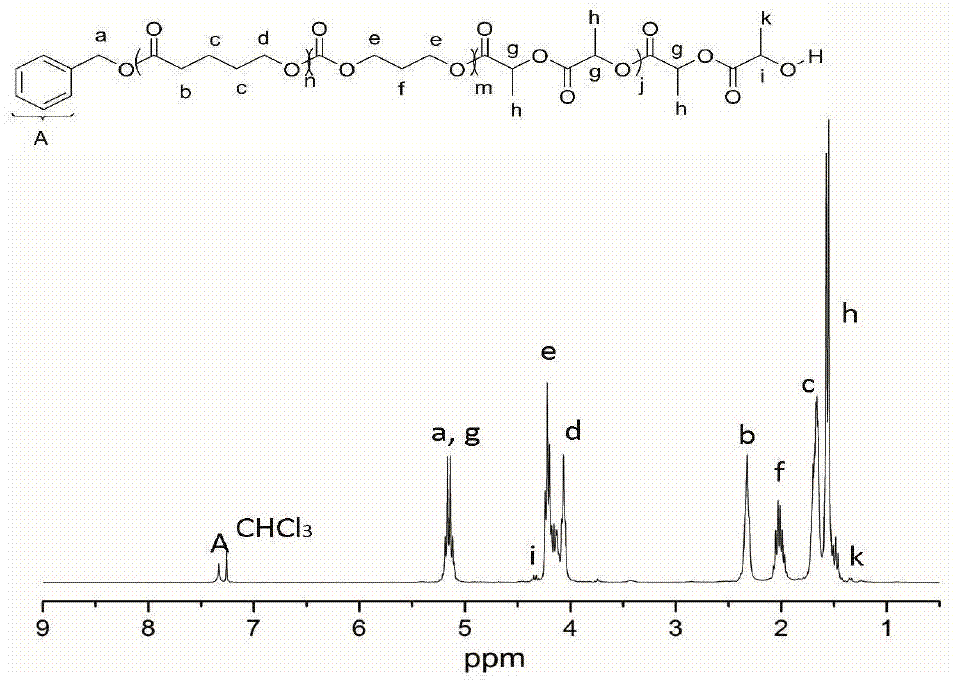
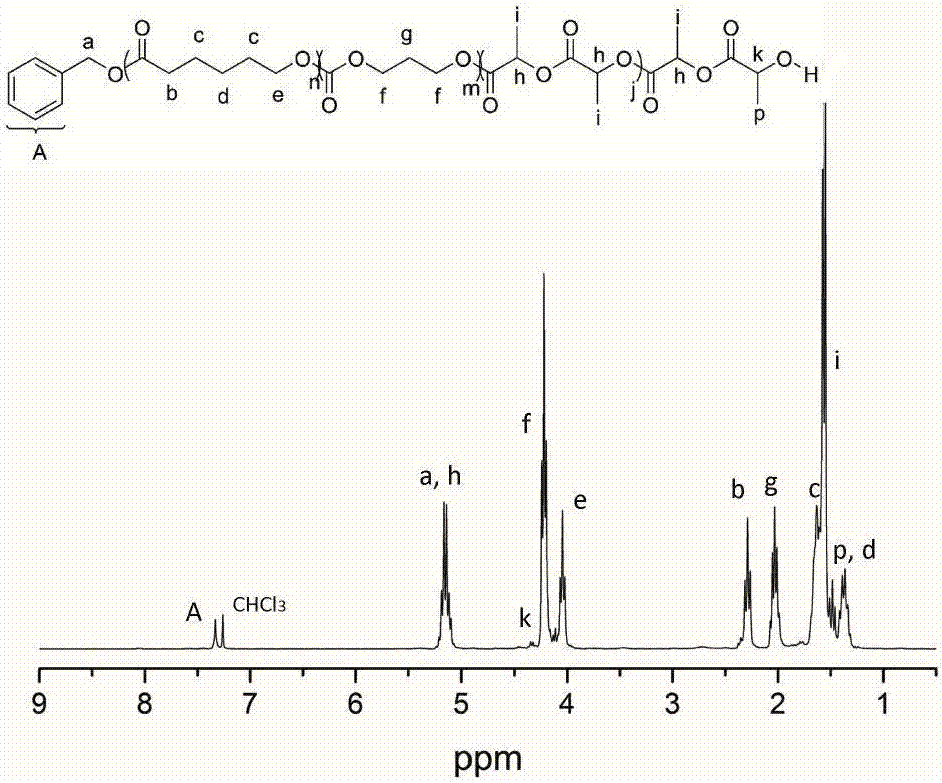
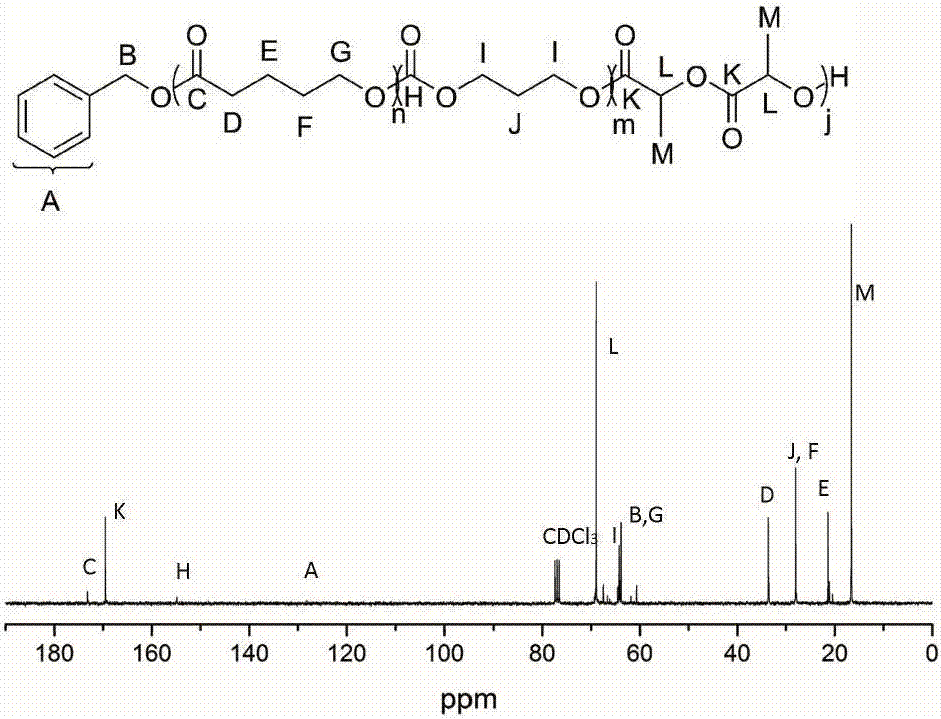
[0040] Add δ-valerolactone (162 μL, 1.8 mmol), DPP (15.0 mg, 60 μmol) into a solution with 2 mL of CH 2 Cl 2 in the polymerization tube. The initiator benzyl alcohol (6.24 μL, 60 μmol) was added into the reaction system to start the polymerization reaction, and the polymerization reaction was carried out at room temperature, and the whole system was under the protection of argon. After the first polymerization reaction was carried out in magnetic stirring for 45 minutes, 1 H NMR detected that δ-valerolactone was substantially consumed, and trimethylene carbonate (183.6 mg, 1.8 mmol) was added to the reaction mixture to start the second block polymerization. When the second block polymerization was carried out for 15 hours, 1 H NMR detection of the second monomer trimethylene carbonate is also basically consumed, then add DBU (17.8 μ L, 120 μ mol) to the mixture, after magnetic stirring for about 10 minutes, add lactide (260 mg, 1.8 mmol) to start the first Tri-block polyme...
Embodiment 2
[0042] Add ε-caprolactone (191 μL, 1.8 mmol), MSA (4.0 μL, 60 μmol) into a solution with 2 mL of CH 2 Cl 2 in the polymerization tube. The initiator benzyl alcohol (6.24 μL, 60 μmol) was added into the reaction system to start the polymerization reaction, and the polymerization reaction was carried out at room temperature, and the whole system was under the protection of argon. After the first polymerization reaction was carried out in magnetic stirring for 1.5 hours, 1 H NMR detected that δ-valerolactone was substantially consumed, and trimethylene carbonate (183.6 mg, 1.8 mmol) was added to the reaction mixture to start the second block polymerization. When the second block polymerization was carried out for 3 hours, 1 H NMR detection of the second monomer trimethylene carbonate is also basically consumed, then add DBU (17.8 μ L, 120 μ mol) to the mixture, after magnetic stirring for about 10 minutes, add lactide (260 mg, 1.8 mmol) to start the first Tri-block polymeriza...
Embodiment 3
[0044] Add β-butyrolactone (144 μL, 1.8 mmol), TfOH (5.14 μL, 60 μmol) into a solution with 2 mL of CH 2 Cl 2 in the polymerization tube. The initiator benzyl alcohol (6.24 μL, 60 μmol) was added into the reaction system to start the polymerization reaction, and the polymerization reaction was carried out at room temperature, and the whole system was under the protection of argon. After the first polymerization reaction was carried out under magnetic stirring for 5 hours, 1 H NMR detected that δ-valerolactone was substantially consumed, and trimethylene carbonate (183.6 mg, 1.8 mmol) was added to the reaction mixture to start the second block polymerization. When the second block polymerization was carried out for 0.5 hours, 1 H NMR detection of the second monomer trimethylene carbonate is also basically consumed, then add DBU (17.8 μ L, 120 μ mol) to the mixture, after magnetic stirring for about 10 minutes, add lactide (260 mg, 1.8 mmol) to start the first Tri-block poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com