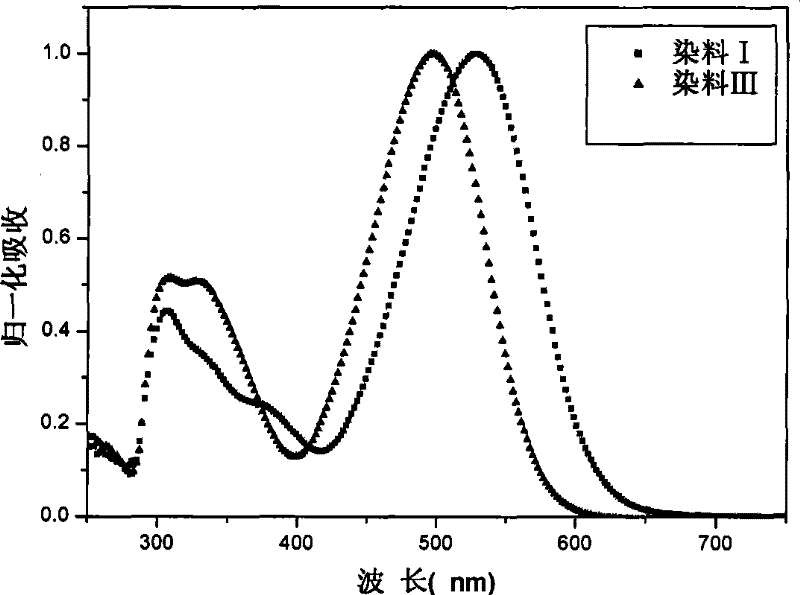
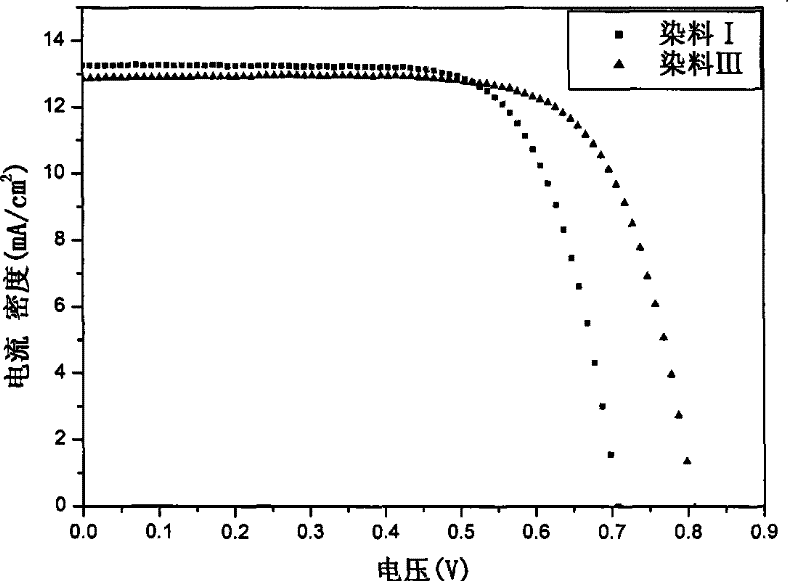
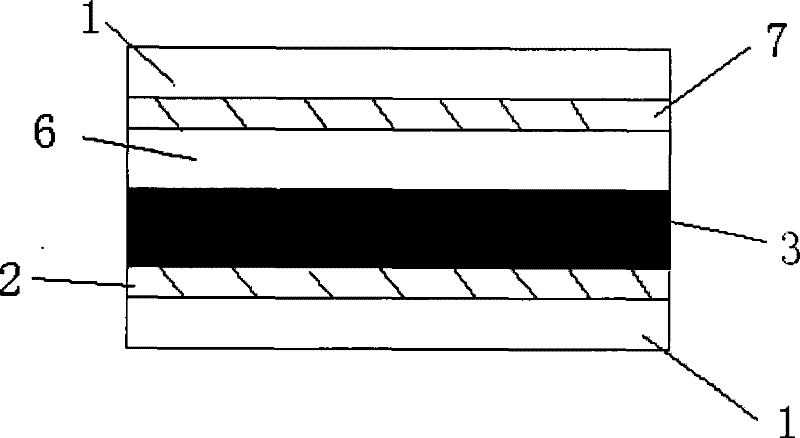
Organic dye with five-element heterocycle and derivative thereof as conjugate unit, and dye sensitization solar cell prepared thereby
A technology of organic dyes and derivatives, applied in the field of dye-sensitized solar cells, can solve problems such as limiting practical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the synthetic route of dyestuff I is as follows:
[0071]
[0072] The specific synthesis method is:
[0073] Dissolve 5.24 g of compound a-1, 5.5 g of 2-selenophenetributyltin, and 0.7 g of bistriphenylphosphine palladium dichloride in 50 mL of THF, reflux the reaction under argon for 12 h, remove the solvent, and perform column chromatography on the residue Compound b-1 was obtained. React 0.57g of compound b-1, 0.2mL of phosphorus oxychloride, DMF and 10mL of chloroform at room temperature for 5h, dilute with chloroform, wash with saturated sodium bicarbonate solution and saturated brine successively, remove the solvent, and obtain the compound by column chromatography c-1. Dissolve 0.5 g of compound c-1, 0.21 g of cyanoacetic acid and 0.6 mL of piperidine in 10 mL of chloroform, reflux for 4 h under argon, remove the solvent, and obtain dye I by column chromatography.
[0074] NMR data of dye I: 1 H NMR (400MHz, DMSO-d 6 )δ: 0.88(t, 6H), 1.30(m...
Embodiment 2
[0076] Using 2,2'-diselenol-5-tributyltin instead of 2-selenophene tributyltin as the starting material, dye II was prepared according to the same experimental process as in Example 1.
[0077] NMR data of dye II: 1 H NMR (400MHz, DMSO-d 6 )δ: 0.88(t, 6H), 1.31(m, 8H), 1.40(m, 4H), 1.68(m, 4H), 3.94(t, 4H), 6.72(d, 2H), 6.91(d, 4H ), 7.04(d, 4H), 7.45(d, 2H), 7.48(d, 1H), 7.49(d, 1H), 7.66(d, 1H), 8.07(d, 1H), 8.40(s, 1H) .
Embodiment 3
[0079] Using 2-tributyltin furan instead of 2-tributyltin selenophene as the starting material, dye III was prepared according to the same experimental process as in Example 1.
[0080] NMR data of dye III: 1 H NMR (400MHz, DMSO-d 6 )δ: 0.88(t, 6H), 1.31(m, 8H), 1.42(m, 4H), 1.70(m, 4H), 3.94(t, 4H), 6.75(d, 2H), 6.92(d, 4H ), 7.08 (d, 4H), 7.11 (d, 1H), 7.51 (d, 1H), 7.70 (d, 2H), 7.982 (s, 1H), 13.48 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com