Enzyme-linked immunosorbent assay for indirectly detecting pirimiphos-methyl
An enzyme-linked immunoassay, pirimiphos-methyl technology, applied in the field of immunoassay, can solve the problems of cumbersome preparation steps and complex hapten structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
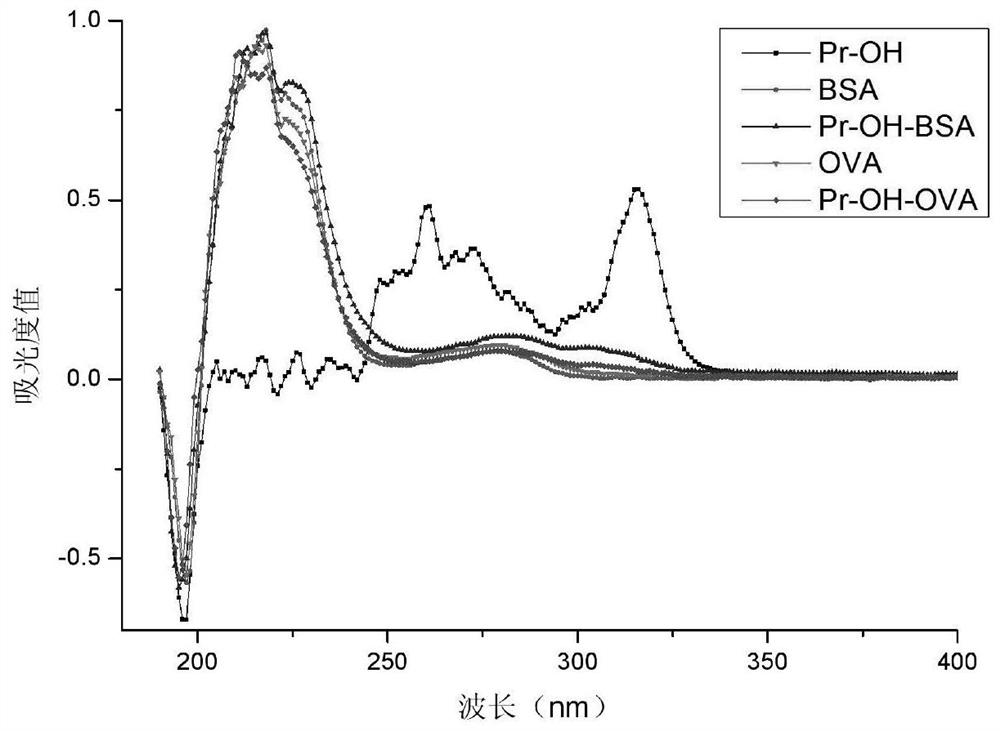
[0042] Embodiment 1 Preparation and Identification of Immunogen and Coating Gen
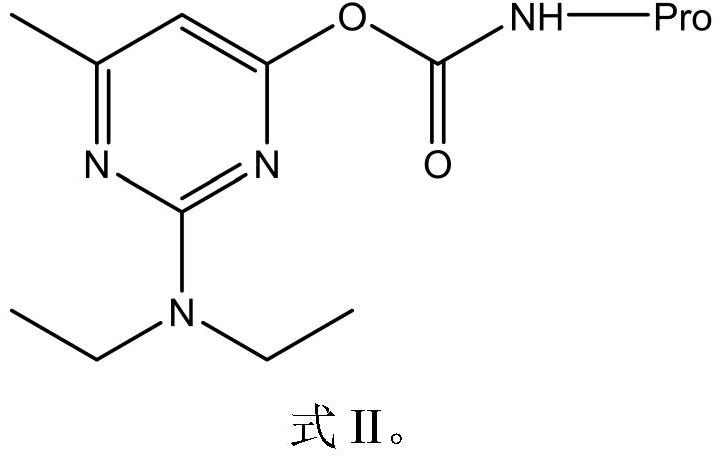
[0043] The preparation of the immunogen and the coating source is realized by using different carrier proteins. The immunogen carrier protein is bovine serum albumin (BSA), the coating original carrier protein is chicken ovalbumin (OVA), and the coupling method adopted is N,N-carbonyldiimidazole method. The specific implementation scheme is as follows:
[0044] Dissolve 0.03mmol of hapten formula I (2-diethylamino-6-methyl-4-pyrimidinol) in 250μL of DMF, add 8.11mg of N,N-carbonyldiimidazole, stir at 37°C for 2h, this is Liquid A. Weigh 10 mg of carrier protein (BSA or OVA) and dissolve it in 2 mL of coating solution, which is B solution. Slowly add solution A to solution B dropwise, stir and react for 72 hours at 4°C. After the reaction, put it into a dialysis bag boiled for 10 minutes in advance, dialyze with PBS for 3 days, and change the dialysate 3 times a day. The obtained complete ant...
Embodiment 2
[0048] Preparation and Identification of Embodiment 2 Antibodies
[0049] 1. Preparation of Polyclonal Antiserum
[0050] Mix the immunogen Pr-OH-BSA prepared in Example 1 with an equal dose of Freund's adjuvant (complete Freund's adjuvant for the first immunization, and incomplete Freund's adjuvant for subsequent immunizations), fully emulsify, and use the back, The mice were immunized by multi-point injections such as abdomen, subcutaneous, and foot pads. Booster immunizations were given every two weeks after the first immunization. Tail blood was collected every other week after the third booster immunization, and the serum titer was determined by indirect competition ELISA. When the titer does not rise any more, intraperitoneal injection is used to boost the immunization once, and the eyeballs are picked out one week later to take blood. Let stand at room temperature for 0.5-1 hour, centrifuge at 12,000 r / min at 4°C for 10 minutes, take the supernatant and put it in a c...
Embodiment 3
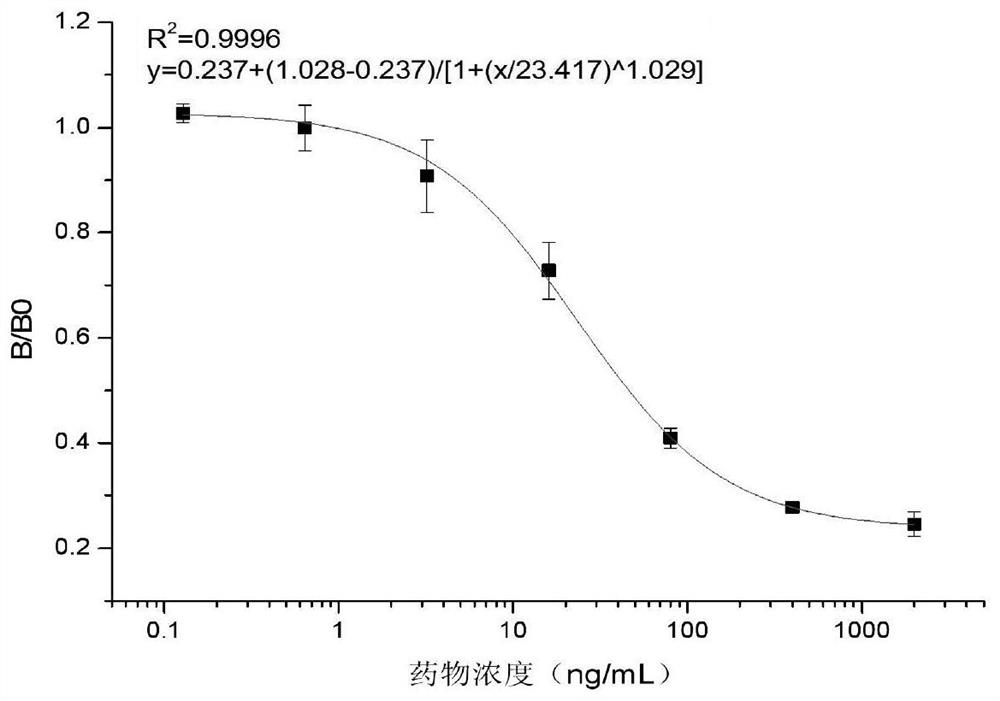
[0054] Example 3 Establishment of an ELISA method for indirect determination of pirimiphos-methyl
[0055] The antiserum prepared in Example 2 was used to draw an indirect competition ELISA standard curve. Use phosphate buffered saline (PBS, 0.1mol / L, pH7.4) as the diluent and reaction buffer of all samples; Use Pr-OH-OVA as the coating former, specific examples are as follows:
[0056] (1) Coat 1 ng / mL of the original Pr-OH-OVA coating on the ELISA microtiter plate. After coating overnight at 37 ° C, add 120 μ L of 5% skimmed milk powder solution per well, block at 37 ° C for 3 hours, and then spin dry. Dry in an oven at 37°C for later use.
[0057] (2) adding sodium hydroxide solution to the sample treatment solution, adjusting the pH of the solution to 11, and shaking at 50° C. for 2 hours to obtain a hydrolyzed sample solution;
[0058] (3) Add 50 μL of a series of concentrations (0.128, 0.64, 3.2, 16, 80, 400, 2000ng / mL) 2-diethylamino-6-methyl-4-pyrimidinol standard so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com