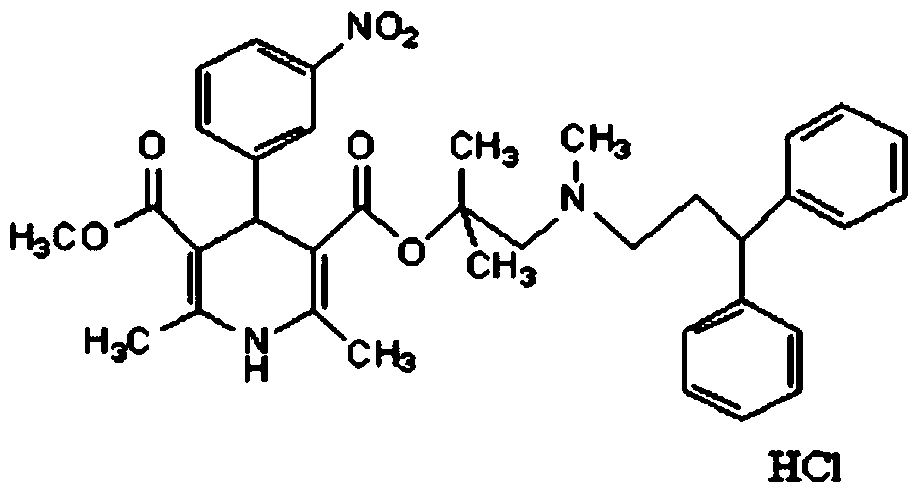
A kind of lercanidipine hydrochloride tablet and preparation method thereof
A technology of lercanidipine hydrochloride and lercadipine hydrochloride, which is applied in the direction of sugar-coated pills, pill delivery, cardiovascular system diseases, etc., can solve the problems of lercanidipine hydrochloride yield and dissolution rate to be improved, high cost of raw materials, etc., to achieve dissolution High yield, high yield, and feasible preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
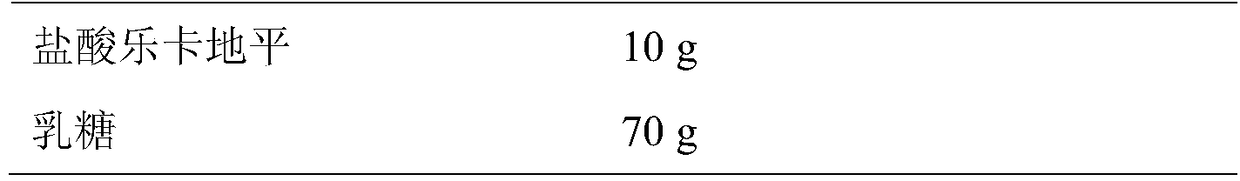
[0020] Prescription 1
[0021]
[0022]
[0023] The preparation process is as follows:
[0024] (1) Pulverize the raw material of lercanidipine hydrochloride, pass through a 100 mesh sieve, pass lactose, microcrystalline cellulose, and sodium carboxymethyl starch respectively through an 80 mesh sieve, dissolve povidone K30 in purified water, and make a mass fraction of 5% povidone K30 solution;
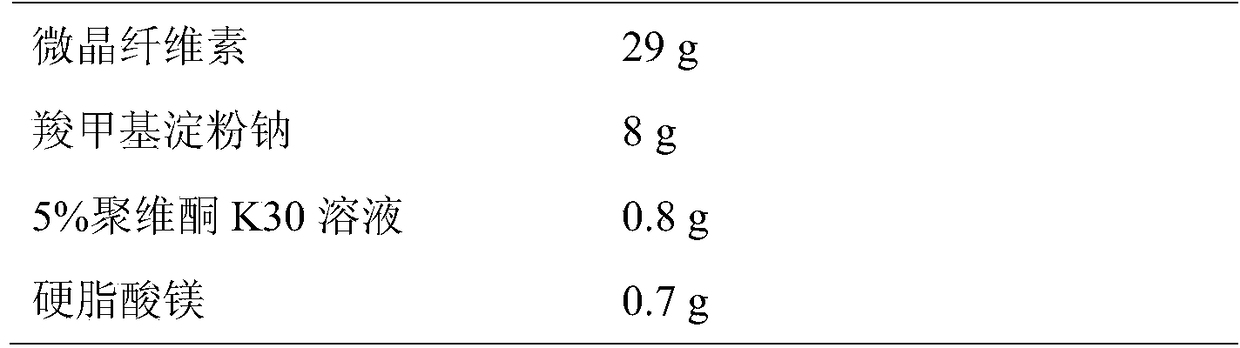
[0025] (2) 10g of lercanidipine hydrochloride and 70g of lactose were mixed uniformly by the method of equal increments, then 29g of microcrystalline cellulose and 8g of sodium carboxymethyl starch were added, placed in a mixer and mixed uniformly;
[0026] (3) Transfer the mixed powder into a swinging granulator, use 0.8g, 5% povidone K30 to make a soft material, pass through a 30-mesh sieve for granulation, and dry at 50°C;
[0027] (4) passing the dry granules through a 30-mesh sieve for granulation, adding 0.7g of magnesium stearate, and mixing uniformly to obtain an inte...
Embodiment 2
[0032] Prescription 2
[0033]
[0034] The preparation process is as follows:
[0035] (1) Pulverize the raw material of lercanidipine hydrochloride, pass through a 100 mesh sieve, pass lactose, microcrystalline cellulose, and sodium carboxymethyl starch respectively through an 80 mesh sieve, dissolve povidone K30 in purified water, and make a mass fraction of 5% povidone K30 solution;
[0036] (2) 10g of lercanidipine hydrochloride and 72g of lactose were mixed uniformly by the method of equal increments, then 31g of microcrystalline cellulose and 9g of sodium carboxymethyl starch were added, placed in a mixer and mixed uniformly;
[0037] (3) Transfer the mixed powder into a swinging granulator, use 1g, 5% povidone K30 to make a soft material, pass through a 30-mesh sieve to granulate, and dry at 50°C;
[0038] (4) passing the dry granules through a 40-mesh sieve for granulation, adding 0.8g of magnesium stearate, and mixing uniformly to obtain an intermediate;
[003...
Embodiment 3
[0043] Prescription 3
[0044]
[0045] The preparation process is as follows:
[0046] (1) Pulverize the raw material of lercanidipine hydrochloride, pass through a 100 mesh sieve, pass lactose, microcrystalline cellulose, and sodium carboxymethyl starch respectively through an 80 mesh sieve, dissolve povidone K30 in purified water, and make a mass fraction of 5% povidone K30 solution;
[0047] (2) 10g of lercanidipine hydrochloride and 73.5g of lactose were mixed uniformly by the method of equal increments, then 31.5g of microcrystalline cellulose and 10g of sodium carboxymethyl starch were added, placed in a mixer and mixed uniformly;
[0048] (3) Transfer the mixed powder into a swinging granulator, use 1.1g, 5% povidone K30 to make a soft material, pass through a 30-mesh sieve to granulate, and dry at 50°C;
[0049] (4) Pass the dry granules through a 50-mesh sieve for granulation, add 1g of magnesium stearate, and mix uniformly to obtain an intermediate;
[0050] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com