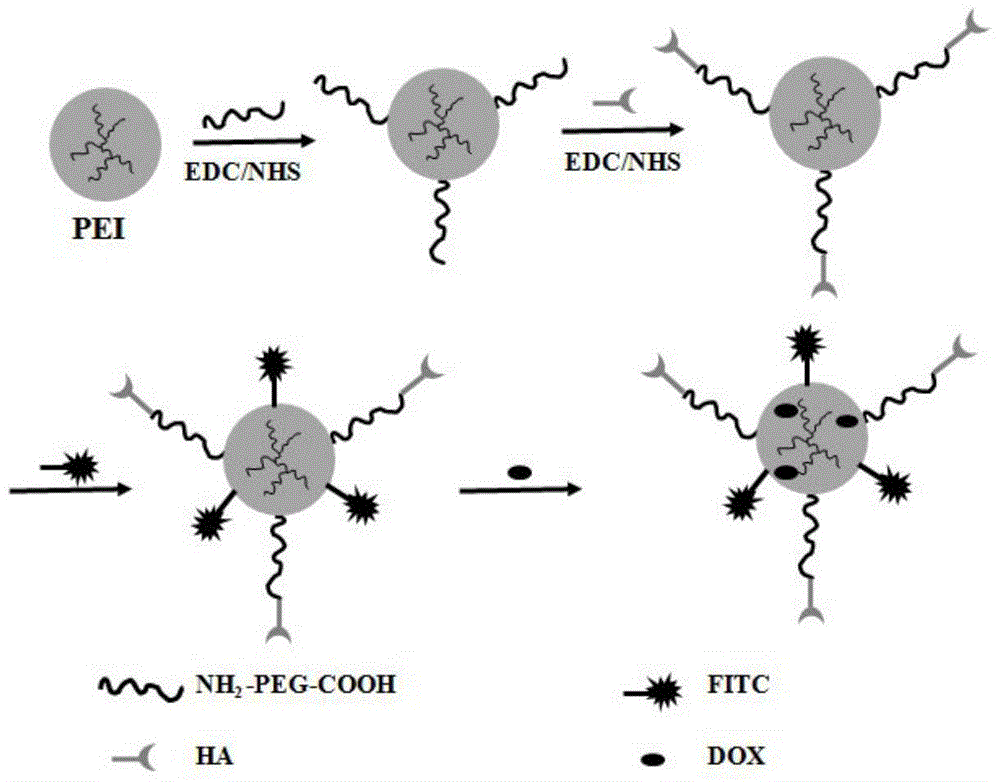
Preparation method of hyaluronic acid-targeted multifunctional branched polyethyleneimine drug carrier
A technology of branched polyethyleneimine and polyethyleneimine, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations of non-active ingredients, etc., to achieve the effect of cost reduction, obvious inhibition effect and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
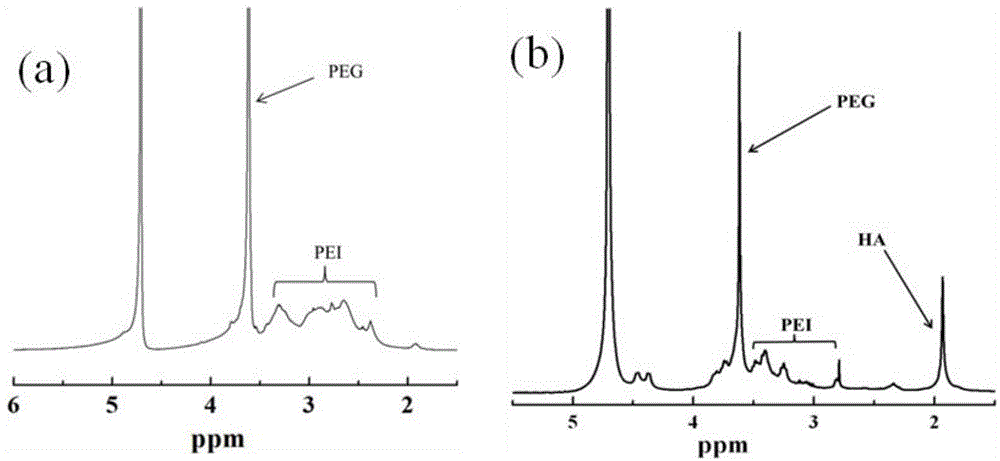
[0046] (1) in 5mLNH 2 - PEG-COOH in DMSO (12 mg / mL), add 5 mL of LEDC in DMSO (7.6 mg / mL), stir for 0.5 hour, then add 5 mL of NHS in DMSO (4.6 mg / mL), continue to stir for 3 hours. Then it was added dropwise to 10 mL PEI in DMSO solution (5 mg / mL), and reacted with rapid stirring for 3 days. The reaction product was dialyzed against PBS buffer solution for 1 day (3×2 L), then deionized water for 2 days (6×2 L), and freeze-dried to obtain white solid powder PEI-PEG.
[0047] (2) In 10mL HA aqueous solution (9.57mg / mL), add 5mL LEDC aqueous solution (3.0mg / mL), stir for 0.5 hours, then add 3mL NHS aqueous solution (2.75mg / mL), and stir for 3 hours. Then it was added dropwise to the above 10 mL PEI-PEG solution in water (2.5 mg / mL), and reacted with rapid stirring for 3 days. The reaction product was dialyzed against deionized water for 3 days (6×2L), and freeze-dried to obtain PEI-(PEG-HA) as a white solid powder.
[0048] (3) Add 2 mL of FI aqueous solution (0.48 mg / mL) dro...
Embodiment 2
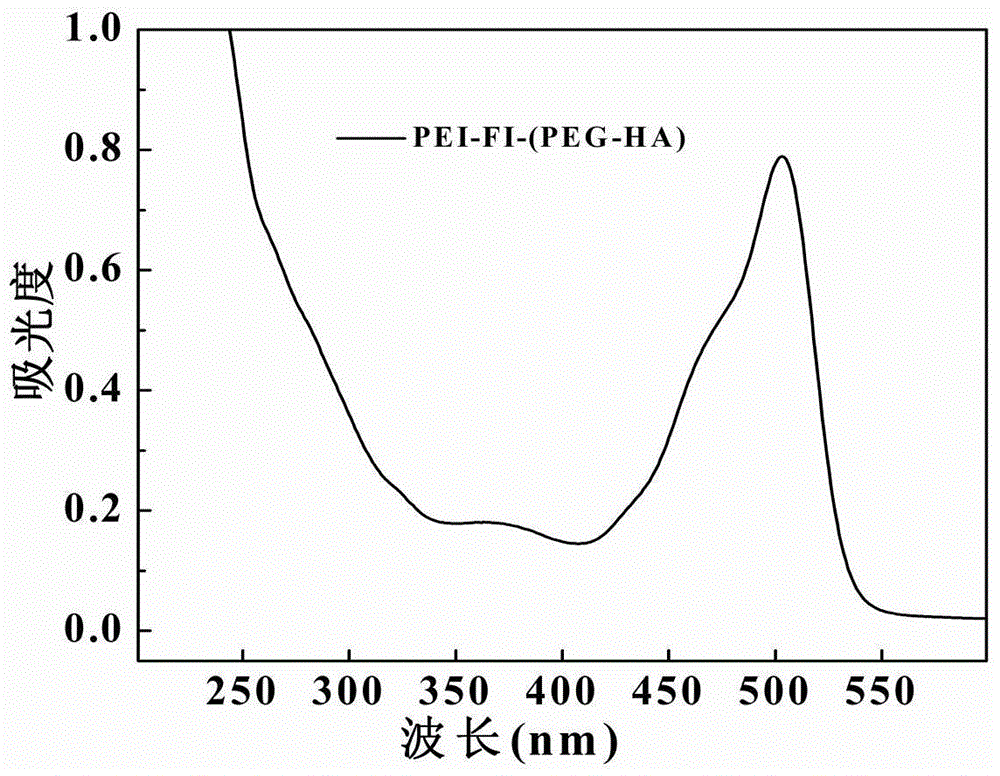
[0055] Add 10 μL triethylamine to 1.7 mg / mL DOX methanol solution, add it dropwise to 10 mL PEI-FI-(PEG-HA) aqueous solution (3.0 mg / mL), and stir at room temperature for 24 hours. After the reaction, the solution was transferred to a 15mL centrifuge tube, centrifuged at 8000rpm for 5 minutes, and the supernatant was lyophilized to obtain the reaction product PEI-FI-(PEG-HA) / DOX. See attached Figure 4 , the supernatant is analyzed by ultraviolet light, and the ultraviolet absorption peak of DOX is located at 480nm. According to the comparison between the ultraviolet absorption value of the prepared nano-drug sustained-release system at 480nm and the DOX standard curve, the content of DOX can be obtained, and the DOX can be calculated. The upload rate is 88%. The molar equivalent of DOX to PEI-FI-(PEG-HA) is 17.5:1.
Embodiment 3
[0057] Dissolve the PEI-FI-(PEG-HA) / DOX prepared in Example 2 with pH=7.0 and pH=5.4 buffers respectively to a solution with a concentration of 1mg / mL, take 1mL and put it into a dialysis bag for fixation, place in Place in a container containing 9mL of buffer solution of different pH and shake in a shaker at 37°C. Samples were taken at 0.5, 1, 2, 3, 5, 8, 12, 16, 24, 36, 48 hour time points. Take 1mL of the liquid outside the dialysis bag each time, measure its absorbance at 480nm, and then add 1mL of the corresponding buffer solution to the outside of the dialysis bag. This method was used to obtain the release curves of DOX released from PEI-FI-(PEG-HA) / DOX under different pH conditions in vitro. See attached Figure 5 , DOX in PEI-FI-(PEG-HA) / DOX is released faster under acidic conditions than under normal conditions, and the release of DOX reaches a stable level after 8 hours of release. The release rate of DOX from PEI-FI-(PEG-HA) / DOX in the weak acidic environment of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com