Magnetically therapeutic antibacterial haemostatic wound dressing and preparation method thereof
A hemostatic dressing, magnetic therapy technology, applied in magnetic therapy, electrotherapy, treatment and other directions, can solve problems such as affecting physical health, limited drug absorption, reducing drug effect, etc., to reduce skin deformity and hyperplasia, increase blood circulation, improve effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
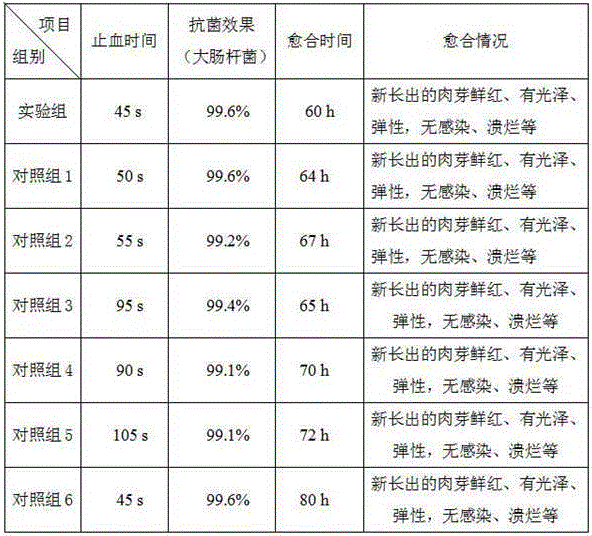
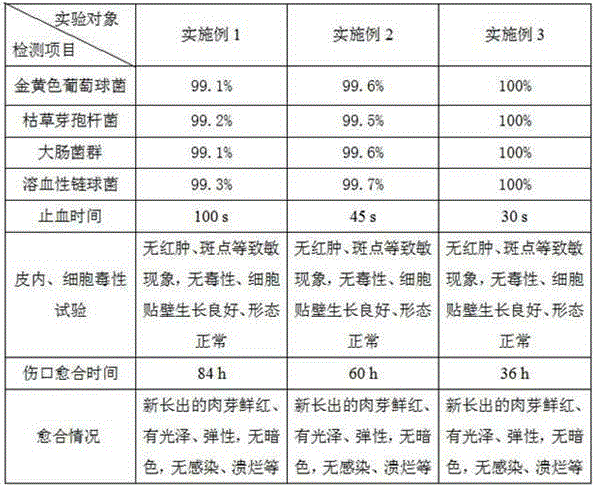
[0032] A magnetic therapy antibacterial and hemostatic dressing, which consists of the following components in parts by weight: 0.5 part of alginate, 0.5 part of chitosan, 0.5 part of crosslinking agent, 0.1 part of permanent magnet nanoparticles, 0.5 part of nano silicon dioxide, Growth factor 0.005 parts. The alginate is sodium alginate, the cross-linking agent is calcium chloride, the deacetylation degree of the chitosan is greater than 95%, and the molecular mass is 10000~20000Da; the permanent magnet magnetic nanoparticles It is a neodymium-iron-stilbene magnet particle, and the magnetic field strength is 0.15-0.35 tesla; the pore size of the nano-silicon dioxide is 20-80 μm; the growth factor is fibroblast growth factor or epidermal growth factor.
[0033] A preparation method of a magnetic therapy antibacterial hemostatic dressing, comprising the steps of:
[0034] S1. Dissolve alginate in purified water, mix and stir for 0.5h~1h until uniform, and prepare solution A; ...
Embodiment 2
[0041]A magnetic therapy antibacterial hemostatic dressing and a preparation method thereof, which consist of the following components in parts by weight: 2 parts of alginate, 3 parts of chitosan, 2 parts of crosslinking agent, 1 part of permanent magnet nanoparticles, and nanometer dioxide Silicon 2 parts, growth factor 0.2 parts. The alginate is sodium alginate, the cross-linking agent is calcium chloride, the deacetylation degree of the chitosan is greater than 95%, and the molecular mass is 10000~20000Da; the permanent magnet magnetic nanoparticles It is a neodymium-iron-stilbene magnet particle, and the magnetic field strength is 0.15-0.35 tesla; the pore size of the nano-silicon dioxide is 20-80 μm; the growth factor is fibroblast growth factor or epidermal growth factor.
[0042] A preparation method of a magnetic therapy antibacterial hemostatic dressing, comprising the steps of:
[0043] S1. Dissolve alginate in purified water, mix and stir for 0.5h~1h until uniform,...
Embodiment 3
[0050] A magnetic therapy antibacterial hemostatic dressing and a preparation method thereof, which consist of the following components in parts by weight: 4 parts of alginate, 6 parts of chitosan, 4 parts of crosslinking agent, 2 parts of permanent magnet nanoparticles, nanometer dioxide Silicon 4 parts, growth factor 0.5 parts. The alginate is sodium alginate, the cross-linking agent is calcium chloride, the deacetylation degree of the chitosan is greater than 95%, and the molecular mass is 10000~20000Da; the permanent magnet magnetic nanoparticles It is a neodymium-iron-stilbene magnet particle, and the magnetic field strength is 0.15-0.35 tesla; the pore size of the nano-silicon dioxide is 20-80 μm; the growth factor is fibroblast growth factor or epidermal growth factor.
[0051] A preparation method of a magnetic therapy antibacterial hemostatic dressing, comprising the steps of:
[0052] S1. Dissolve alginate in purified water, mix and stir for 0.5h~1h until uniform, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Magnetic field strength | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com