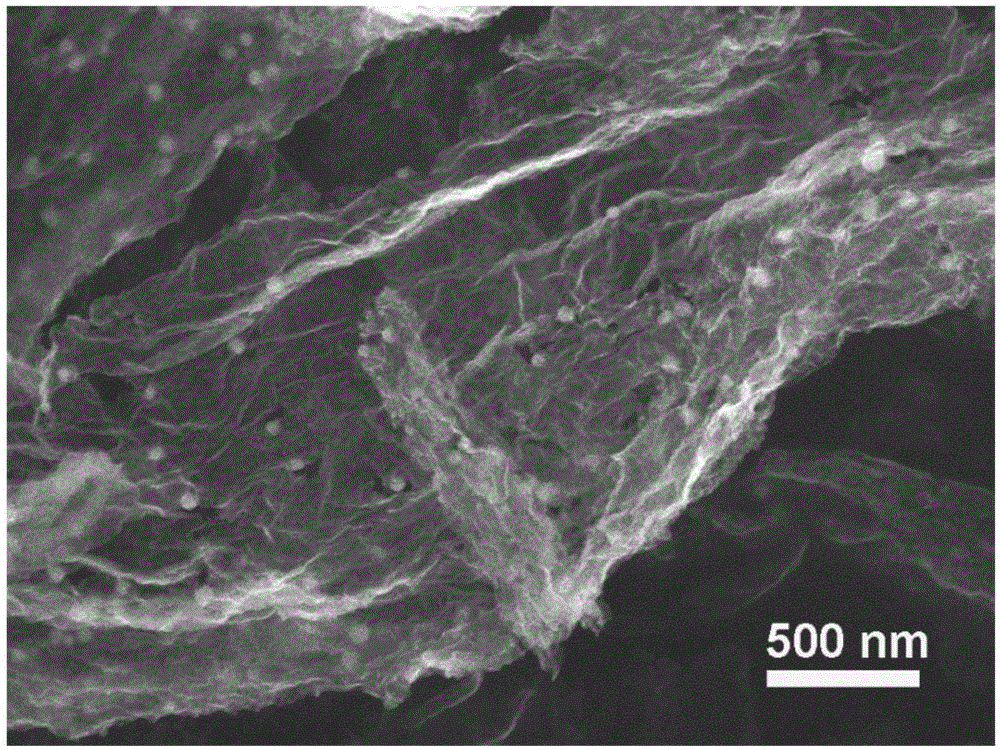
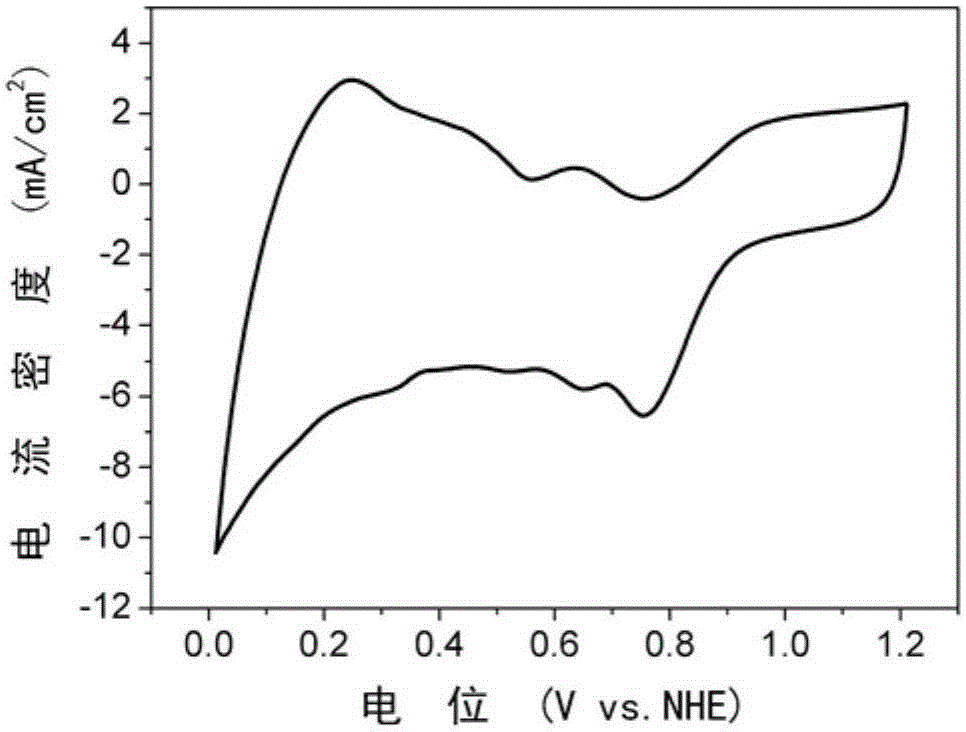
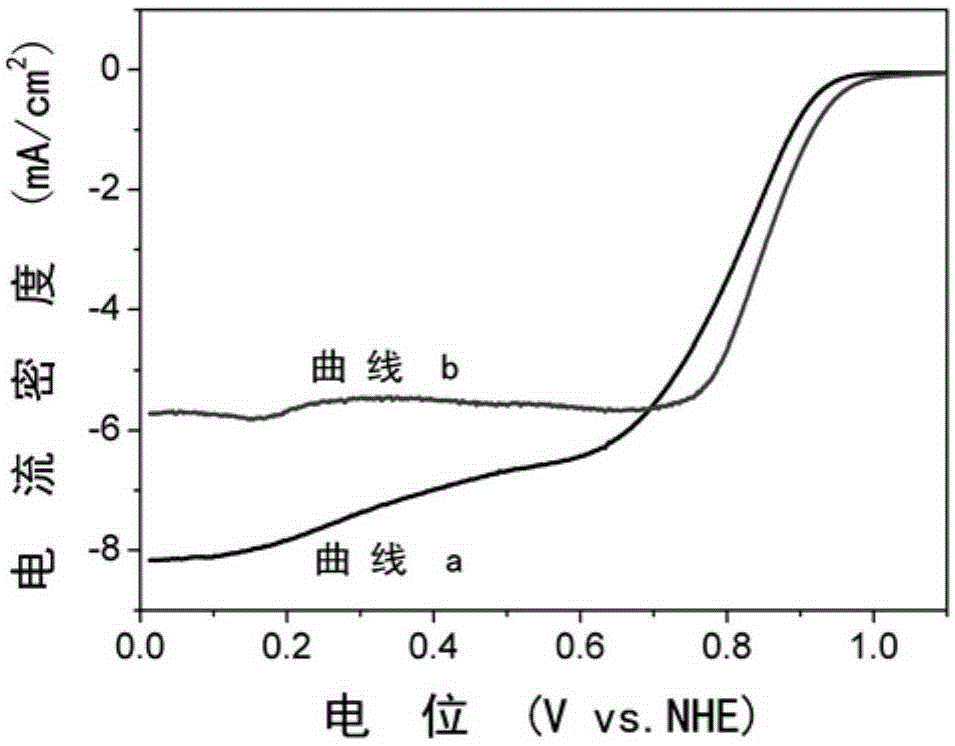
Nitrogen-doped graphene-iron-based nanoparticle composite catalyst and preparation method thereof
A nitrogen-doped graphene and nanoparticle technology is applied in the field of electrochemical catalysis to achieve the effects of good cycle stability, excellent methanol tolerance, and high oxygen reduction catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step 1: Weigh 80 mg of graphene oxide (Zhongke Times Nano, Chengdu Organic Chemical Co., Ltd.), ultrasonically disperse it in 400 mL of deionized water, and prepare a graphene oxide aqueous solution with a concentration of 0.2 mg / mL. The above graphene oxide aqueous solution was placed in a 1000 mL three-necked flask, 100 μL of hydrazine hydrate solution (80% by mass fraction) was added, and reacted in an oil bath at 95° C. for 1 hour (full magnetic stirring). After the solution was cooled, it was filtered to remove large pieces of reduced graphene oxide to obtain a black water dispersion of reduced graphene oxide (concentration was about 0.15 mg / mL).
[0028] Step 2: Weigh 25 mg of ferric chloride hexahydrate, add it to the above-mentioned reduced graphene oxide dispersion, and stir thoroughly for 12 hours to obtain a mixed solution (the mass ratio of iron content to reduced graphene oxide is about 1:12) , The airgel precursor was obtained after freeze-drying at -62°C....
Embodiment 2
[0032] Step 1: Weigh 200 mg of graphene oxide (Zhongke Times Nano, Chengdu Organic Chemical Co., Ltd.), ultrasonically disperse it in 200 mL of deionized water, and prepare a graphene oxide aqueous solution with a concentration of 1 mg / mL. The graphene oxide aqueous solution was placed in a 500 mL three-necked flask, 800 mg of sodium borohydride was added, and after fully magnetically stirred for 3 hours, it was reacted in a 95° C. oil bath for 1 hour (fully magnetically stirred). After the solution is cooled, filter to remove large pieces of reduced graphene oxide; rinse with a large amount of deionized water to remove residual ions. Finally, a black water dispersion of reduced graphene oxide (concentration about 0.5 mg / mL) was obtained.
[0033] Second step: take by weighing 84mg ferric oxalate pentahydrate, join in the above-mentioned reduced graphene oxide dispersion liquid, obtain mixed solution (the mass ratio of iron content and reduced graphene oxide is about 1:5) afte...
Embodiment 3
[0036] Step 1: Weigh 120 mg of graphene oxide (Zhongke Times Nano, Chengdu Organic Chemical Co., Ltd.), ultrasonically disperse it in 400 mL of deionized water, and prepare a graphene oxide aqueous solution with a concentration of 0.3 mg / mL. The above graphene oxide aqueous solution was placed in a 1000 mL three-necked flask, 150 μL of hydrazine hydrate solution (80% by mass fraction) was added, and reacted in an oil bath at 95°C for 1 hour (full magnetic stirring). After the solution was cooled, it was filtered to remove large pieces of reduced graphene oxide to obtain a black water dispersion of reduced graphene oxide (concentration was about 0.25 mg / mL).
[0037]Second step: take by weighing 90mg ferric nitrate nonahydrate, join in the above-mentioned reduced graphene oxide dispersion liquid, obtain mixed solution (the mass ratio of iron content and reduced graphene oxide is about 1:8) after fully stirring 12 hours, in Airgel precursors were obtained after freeze-drying at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com