Method for producing 2,5-dimethyl furan (2,5-DMF) by fructose one-step process
A technology of dimethylfuran and dimethylformamide, which is applied in the field of one-step preparation of 2,5-DMF, can solve the problems of difficulty in separating 5-HMF, poor selectivity, etc., and achieves good reusability and simple and easy experimental operation. The effect of large industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 fructose one-step method produces 2,5-DMF
[0021] The experiment was carried out in a steel autoclave with a volume of 100mL, the amount of raw material fructose was 1.8g (10mmol), and the catalyst Ru / C, AlCl 3 , H 2 SO 4 , H 3 PO 4 The dosage is 0.2g, 0.056g (0.42mmol), 0.098g (1mmol), 0.147g (1.5mmol), the solvent DMF dosage is 60mL, the control speed is 600rpm, the hydrogen pressure is 1.5MPa, the reaction temperature is 200°C, the reaction is 12h, the reaction After the end, take samples directly, use HPLC and gas chromatography to detect, filter the reaction liquid, recover Ru / C from the filter cake, wash Ru / C with DMF, and recycle, add a few grains of zeolite to the filtrate, at 96 ° C, normal pressure conditions The rectification operation was carried out at a lower temperature to obtain a colorless oily liquid. The detection and analysis showed that the yield of 2,5-DMF was 58.5 mol%, and the conversion rate of fructose was >99 mol%.
[0022] ...
Embodiment 2-12
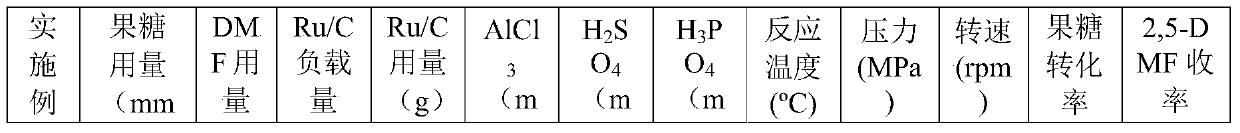
[0025] Referring to the test conditions described in Table 1, the method in Example 1 was used to prepare 2,5-DMF from fructose in one step, and the results are shown in Table 1.
[0026] Table 1 embodiment 2-12 test conditions and results
[0027]
[0028]
Embodiment 13-15
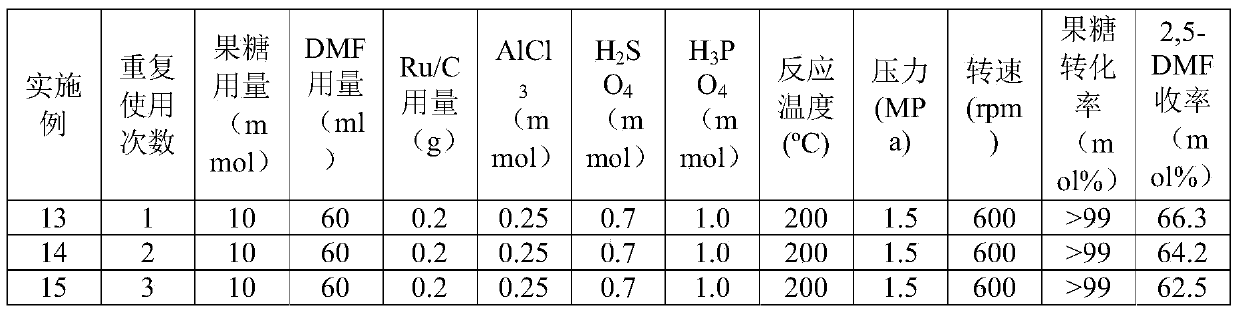
[0030] According to the test conditions described in Table 2, the method described in Example 1 was used to conduct the test with the Ru / C recovered in Example 15. The test conditions and results are shown in Table 2.
[0031] Table 2 Ru / C recycling situation
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com